FDA Investigator CDR Ileana Barreto Pettit
CDR Ileana Barreto Pettit has conducted inspections on 308 sites in 21 countries as of 05 Dec 2022. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
308
Last Inspection Date:
05 Dec 2022
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
Puerto Rico,
United States of America,
China,
Germany,
Italy,
Taiwan,
Portugal,
Australia,
France,
Japan,
Spain,
Czechia,
India,
Ireland,
Finland,
Netherlands,
United Kingdom of Great Britain and Northern Ireland,
Denmark,
Canada,
Sweden,
Brazil
FDA Investigators that have inspected at least one site in common with CDR Ileana Barreto Pettit:
Aaron F Coleman,
Aaron J Fox,
Adalberto Cajigas,
Adaliz Santaliz Cruz,
Adam R Cooke,
Ademola O Daramola,
Adriana N Jimenez Lopez,
Alan A Rivera,
Albert A Salvador,
Alex J Wild,
Alicia M Mozzachio,
Alison N Stieg,
Allen F Hall,
Althea A Williams,
Amber G Wardwell,
Amir Alavi,
Ana Delp Cintron,
Ana P Barido,
Anabel Veiga,
Anastasia I Onuorah,
Anastasia M Shields,
Andrea D Charles Julien,
Andrea H Norwood,
Andrew J Barrowcliff,
Angela E Glenn,
Angelica M Hernandez,
Anita Narula, PhD,
Ann L Demarco,
Anna Lazar,
Annette Melendez,
Anthony A Charity,
Anthony Abel,
Anthony F Lorenzo,
Ariel Cruz Figueroa,
Arlene M Badillo,
Arsen Karapetyan,
Ashar P Parikh,
Ashley A Mutawakkil,
Ashley B Jelonek,
Atul J Agrawal,
Azza Talaat,
Barbara Janine Breithaupt,
Barbara Jwilimczyk Macri,
Barbara T Carmichael,
Benita C Okeke,
Bill Tacket, Jr,
Bingchen Du, PhD,
Binh T Nguyen,
Bo Chi, PhD,
Bonita S Chester,
Brandon C Heitmeier,
Brett R Havranek,
Brian R Yaun,
Brittani N Franklin,
Brittny C Cargo,
Brooke K Higgins,
Brooke K Seeman,
Brunilda Torres,
Burnell M Henry,
Byungja E Marciante,
Candice C Mandera,
Candice J Cortes,
Capt Apilar Barido,
Carl Lee,
Carla A Norris,
Carla J Lundi,
Carlos A Medina,
Caroline Strasinger,
Carolyn A Renshaw,
Cassandra L Abellard,
Catherine J Laufmann,
CDR Rochelle B Young, RPh, MSA,
CDR Sean T Creighton,
Chaltu Nwakijra,
Chantae D Mitchell,
Charisse K Green,
Charles R Cote, RIC,
Cheryl A Clausen,
Cheryl D Mccall,
Cheryl Love Harris,
Chiaochun J Wang,
Christian D Lynch (CDL),
Christie A Soto,
Christine W Twohy,
Christopher T Smith,
Christos G Tsingelis,
Chyla T Hunter,
Claire M Minden,
Claudette D Brooks,
Clinton J Lott,
Colleen F Hoyt,
Colleen M Aspinwall,
Conner N Mann,
Courtney A Gilbert,
Craig A Garmendia,
Cynthia J Lee, MS,
Cynthia Jim, CSO,
Cynthia M Goudeau,
Damaris Y Hernandez,
Dandan Wang, PhD,
Daniel C Skirvin,
Daniel J Roberts,
Daniel L Aisen,
Danielle S Pearson,
Darryl T Newell,
Daryl A Dewoskin,
David A Oluwo,
David G Eng,
David J Gomes,
David M Beltran,
David M Wilkinson,
Dawn M Mccabe,
Dayna I Martinez,
Deborah A Greco,
Dell S Moller,
Denise M Digiulio,
Dennis Cantellops Paite,
Dennis L Doupnik,
Diane L Raccasi,
Diane T Bargo,
Diane T O'brien,
Dianiris C Ayala,
Dillard H Woody, Jr,
Dionne D Arline,
Dipti Kalra,
Djamila Harouaka,
Dongping Dai, PhD,
Douglas A Campbell,
Douglas C Kovacs,
Dr. Carmelo Rosa,
Dr. Dominick Roselle,
Dr. Gang Wang, PhD,
Dr. Robert C Horan, MD,
Dusty F Snoeberg,
Dyvette Arline,
Edmund F Mrak, Jr,
Edward H Maticka,
Edwin Martinez,
Eileen A Liu,
Elizabeth Ll Edwards,
Ellen C Weisensel,
Emest F Bizjak,
Emily J Orban,
Eric S Weilage,
Erika V Butler,
Erin D Mccaffery,
Ethan P Stegman,
Eva I Merced Medina,
Fabian Nchaparro Rodriguez,
Farhana Khan,
Feiyan Jin,
Felix Maldonado,
Frances L Dejesus,
Frederick L Fricke,
Gabriel E Muniz,
Gam S Zamil,
Garcial,
Gene R Gunn,
George J Flynn,
Gerald B Seaborn, Jr,
Gerardo Z Vazquez,
German Rivera,
Gideon N Esuzor,
Gina C Chen,
Gretchen M Laws,
Guerlain Ulysse,
Gwyn G Dickinson,
Haley H Seymour,
Harshal J Desai,
Hector Jcolon Torres,
Henry K Lau,
Holly Brevig,
Holly M Scott,
Humphrey,
Iraida Ortiz,
Iram R Hassan, PhD,
Israel Santiago, OCM,
Ivan E Reyes,
Ivis L Negron,
J'maica J Hunter,
Jacqueline Mdiaz Albertini,
Jaime E Pares,
James C Maclaughlin,
James I Giefer,
James M Mason,
James M Simpson,
James R Evans,
James W Plucinski,
Jana L Caylor,
Janet A Rajan,
Janet L Bowen,
Jason D Tenney,
Jason F Chancey,
Jason K Morgan,
Jason P Aun,
Javier O Vega,
Jawaid Hamid,
Jay T Wong,
Jean A Peeples,
Jeffery A Hangartner,
Jeffery B Medwid, PhD,
Jeffrey A Sommers,
Jeffrey M Watson,
Jeffrey P Raimondi,
Jeffrey R Wooley,
Jennifer A Robinson,
Jennifer D Hollstrom,
Jennifer L Huntington,
Jennifer Lalama,
Jennifer M Gogley,
Jennifer M Menendez,
Jessica L Pressley,
Jessica P Mcalister,
Jingbo Xiao,
Joann Walters Salvatierra,
Joanne E King,
Joe X Phillips,
Joey V Quitania,
Jogy George,
John A Gonzalez,
John B Crowe,
John J Bernal,
John P Mistler,
John W Banks,
Johnetta F Walters,
Johnna L Bleem,
Jonathan W Chapman,
Jorge E Martinez,
Jorge L Gonzalez,
Jorge L Guadalupe,
Jorge L Lajara,
Jose A Lopez,
Jose A Moreno,
Jose Acruz Gonzalez,
José E Meléndez,
Jose F Pedro,
Jose M Cayuela,
Jose M Gonzalez,
Jose Martinez, Jr,
Jose Perez Soto,
Jose R Hernandez,
Jose R Lopez,
Jose R Rodriguez,
Jose Velez,
Joseph Kutze, PhD,
Joshua J Silvestri,
Joshua P Wireman,
Juan Rjimenez Garcia,
Juanita P Versace,
Juarbe,
June P Page,
Justin A Boyd,
Kara D Dobbin,
Karen C Daugherty,
Karen E D'orazio,
Karen G Hirshfield,
Karen M Rodriguez,
Karissa J Mccaw,
Karl D Hezel,
Katelyn Astaub Zamperini,
Katherine Szestypalow,
Kathleen D Culver,
Kathleen M Sinninger,
Kathryn E King,
Kayla V Sprague,
Keith O Webber, PhD,
Keith Webber, PhD,
Kejun Cheng,
Kellia N Hicks,
Kelly I Anderson,
Kelvin Cheung,
Kemejumaka N Opara,
Kenneth H Williams,
Kenneth Nieves,
Kent C Faul,
Kevin T Nguyen,
Kham Phommachanh,
Kim Lthomas Cruse,
Kimberley A Hoefen,
Ko U Min,
Kouros Kangarli,
Kristen D Evans,
Kristina L Conroy,
Kristy E Zuroski,
Kshitij A Patkar,
Ladislav Kermet,
Laiza V Garcia,
Lance Mde Souza, MBA,
Lareese K Thomas,
Latorie S Jones,
Laura E Garcia,
Laura L Staples,
Laurie A Haxel,
Laurie Graham,
Laurimer Kuilan Torres,
Lawrence J Stringer,
LCDR Angelica M Chica,
LCDR Debra Emerson,
LCDR Michael H Tollon,
LCDR Randall L Morris,
Leroy Terrelonge,
Lesley K Satterwhite,
Leslie A Cartmill,
Leyden Guerrero,
Liatte Kreuger, PharmD,
Libia M Lugo,
Lillian Mcolon Mcknight,
Lillie D Witcher,
Linan Ha, PhD,
Linda F Murphy,
Linda K Matheny,
Linda Linh N Adams,
Linda Thai,
Lindsay R Hatch,
Lindsay R Mundy,
Lisa A Warner,
Lisa M Lopez,
Lisa Sg Shelton,
Lissette Paiz,
Lizaida E Rodriguez,
Lorie S Hannappel,
Lourdes Andujar,
Lt Bill Tackett, Jr,
LT Daveta L Bailey,
LT Kimberly M Hull,
LT Luis O Rodriguez,
LT Prince D Brown,
LT Rafael Gonzalez,
LT Richard A Lyght,
LT Tamara J Henderson,
Lucas B Leake,
Lucila B Nwatu,
Luis A Carrion,
Luis A Dasta,
Luis Mburgos Medero,
Luis Soto Lopez,
Lundy H Patrick,
Lura D Baquero,
Luz I Collado,
Lydia I Rosas Marty,
Lynne Ensor,
Mabany Lizardi,
Marc Balzarini,
Marcus A Ray,
Margaret L Bolte,
Margaret M Annes,
Maria A Ruttell,
Maria V Price,
Marian E Ramirez,
Marianela Aponte Cruz,
Maribel V Juarbe,
Maridalia Torres Irizarry,
Marie F Morin,
Marie T Falcone,
Marinee Flores Marrero,
Marisol Faberlle,
Mariza M Jafary,
Mark I Kaspar,
Mark Sassaman,
Mark W Rivero,
Marshalette O Edwards,
Marta E Gonzalez,
Martin F Muckenfuss,
Martrice A Packer,
Mary A Millner,
Mary E Storch,
Mary F Bodick,
Marybet Lopez Negron,
Massoud Motamed,
Matthew B Casale,
Matthew B Thomaston,
Matthew F Duff,
Medina,
Megan T Ziegler,
Meghan Murphy,
Melanie G Warzala,
Melanie M Walker,
Melanie W Pishnery,
Melissa J Garcia,
Melissa T Roy,
Meredith M Andress,
Meredith M Cobb,
Merril E Racke,
Michael A Charles,
Michael C Lombardi,
Michael E Maselli,
Michael J Nerz,
Michael R Goga,
Michael T Cyrus,
Michele L Glendenning,
Michele L Obert,
Michele Perry Williams,
Michelle K Cockrell,
Michelle Marsh,
Michelle Rfrazier Jessen, PhD,
Michelle S Dunaway,
Miguel A Martinez Perez,
Miguel Gmanzano Maldonado,
Mihaly S Ligmond,
Milos Dokmanovic, PhD,
Minh D Phan,
Mizanne E Lewis,
Monica Cburgos Garcia,
Moraima Jramos Valle,
Mra Davism,
Mra M Barbosar,
Mra Mcculloughj,
Muralidhara B Gavini, PhD,
Myriam M Sosa,
Nakesha J Jackson,
Nancy M Espinal,
Nancy N Bellamy,
Nancy Rosado,
Natalia Pripuzova, PhD,
Nawab A Siddiqui,
Nebil A Oumer,
Neisa M Alonso,
Nicholas A Violand,
Nicholas L Hunt,
Nicholas P Diorio,
Nicolas Riveratorres,
Nicole E Knowlton,
Nicole M Bell,
Noreen Muñiz,
Omayra Nrodriguez Ruiz,
Ormond,
Otto D Vitillo,
Pablo Feliciano,
Parul M Patel,
Patrice S Hall,
Patricia F Hughes, PhD,
Patricia H Dlugosz,
Patrick C Klotzbuecher,
Patrick L Wisor,
Patsy J Domingo,
Paul A Bonneau,
Paul A Licata,
Paul H Dakin,
Paul L Figarole, Jr,
Paula J Bretz,
Peter E Baker,
Peter R Lenahan,
Philip F Istafanos, DMV, MS,
Pratik S Upadhyay, DDC,
Qin Xu,
Qing Joanna Zhou, PhD,
Rabin N Ghoshal,
Rachel A Gomez,
Rachel A Peters,
Rachel C Harrington,
Rafael Nevarez Nieves,
Rafael Padilla,
Ramesh Sood,
Ramon A Hernandez,
Randy L Self,
Raquel Gonzalez Rivera,
Ravindra K Kaallwal,
Raymond A Lyn, Jr,
Raymond T Oji,
Rebecca E Dombrowski,
Rebecca Parrilla,
Rebecca Rodriguez,
Renee S Blosser,
Reyes Candau Chacon, PhD,
Reynolds,
Richard E Needham,
Richard K Vogel,
Richard L Friedman,
Richard L Rutherford,
Robert C Coleman,
Robert C Steyert,
Robert D Tollefsen,
Robert J Ham,
Robert J Maffei,
Robert Sharpnack,
Robin Levis, PhD,
Rochelle K Kimmel,
Rochelle L Cross,
Rodney G Raiford,
Rodney W Lenger,
Roger F Zabinski,
Ronald Ifraimov,
Ronald T Weber,
Ronan F King,
Ronnie E Jackson,
Rosa J Motta,
Rosario D'costa,
Rose Xu,
Roy R Rinc,
Rozelle G Smith,
Russell J Glapion,
Russell K Riley,
Ruth Moore, PhD,
S Lori Brown, PhD MPH,
Saleem A Akhtar,
Salvatore N Randazzo,
Samantha E Cleek,
Samina S Khan,
Sangeeta M Khurana, PhD,
Santos E Camara,
Sarah E Mcmullen,
Sarah E Rhoades,
Sarah Ibrahim,
Satheesh Thomas,
Saundrea A Munroe,
Scott B Laufenberg,
Scott N Lim,
Scott T Ballard,
Sean R Marcsisin,
Sergio E Delgado,
Sharmista Chatterjee,
Sharon Giamberini,
Sharon K Thoma, PharmD,
Shavon L Square,
Shelby N Turner,
Shelly A Rios Laluz,
Sherbet L Samuels,
Sheri L Stephenson,
Sidney B Priesmeyer,
Simone E Pitts,
Siramad Sainz Trujillo,
Sixto M Mercado Rios,
Sneha S Patel,
Song Y Lavalais,
Sonia M Monges,
Sonya M Edmonds,
Stacey F Allard,
Stacey F Rivette,
Stacie A Woods,
Stanley B Eugene, BS, BME,
Stephanie A Slater, MS,
Stephanie D Crockett,
Stephen D Brown,
Steven A Brettler,
Steven B Barber,
Steven D Kehoe,
Steven J Rush,
Steven J Thurber,
Steven M Weinman,
Steven P Donald,
Susan M Corrales,
Susan M Jackson,
Susan M Turcovski,
Susan O Oladeji,
Susan P Bruederle,
Susan T Hadman,
Susan W Ting,
Susanne M Richardson, MS RAC,
Suzanne N Vallez,
Swati Verma,
Tajah L Blackburn,
Tamara M Casselman,
Tamika White,
Tamil Arasu, PhD,
Tammara A Stephens,
Ted L Anderson,
Temar Q Williams,
Teresa I Navas,
Thai D Truong,
Thomas E Friel,
Thomas J Arista,
Thomas R Withers,
Thuy T Nguyen, LCDR,
Timothy T Kapsala,
Todd M Stankewicz,
Tonya R Johnson,
Toyin B Oladimeji,
Tracy J Washington,
Truong Xuan Nguyen (Andy),
Uduak M Inokon,
Uttaniti Limchumroon (Tom),
Victor Spanioli,
Vidya B Pai,
Vilmary Negron Rodriguez,
Vincent Thomas,
Virginia L Meeks,
Vivin George,
Walden H Lee,
Wanda B Coats,
Wanda J Torres,
Wayne D Mcgrath,
Wendy G Tan, PhD,
William A Warnick,
William L Bargo,
William V Millar,
Xiaohui Shen,
Xiaokuang Lai, PhD,
Xiujing L Baggett,
Yasamin Ameri,
Youmin Wang,
Yuesheng Ye,
Yumi J Hiramine,
Yvette I Henry,
Yvette I Johnson,
Zakaria Wahba,
Zumera B Ajani
CDR Ileana Barreto Pettit's Documents
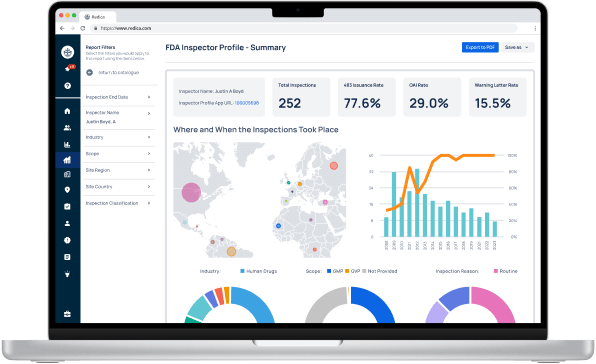
Experience Redica Systems’ NEW investigator profiles and dashboards
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more