FDA Investigator Satheesh Thomas
Satheesh Thomas has conducted inspections on 174 sites in 21 countries as of 01 Nov 2018. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
174
Last Inspection Date:
01 Nov 2018
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
Brazil,
France,
India,
Taiwan,
Switzerland,
Netherlands,
Poland,
United Kingdom of Great Britain and Northern Ireland,
China,
Canada,
Ireland,
Belgium,
Japan,
Italy,
Germany,
Israel,
Korea (Republic of),
Singapore,
Portugal
FDA Investigators that have inspected at least one site in common with Satheesh Thomas:
Ademola O Daramola,
Affiong Flores,
Alan P Kurtzberg,
Alia Legaux,
Alice S Tsao,
Althea A Williams,
Amber D Brodas,
Amy Wyan Mai,
Anastasia M Shields,
Andrew J Garufi,
Anissa M Cheung,
Ankur C Patel,
Ann B Okon,
Anthony A Charity,
Anthony C Warchut,
Anthony J Donato,
Anthony Kovalenko,
Anthony R Rauert,
Arduino Frankovic,
Ariel Cruz Figueroa,
Arindam Dasgupta, PhD,
Arthur F Walker,
Arthur S Williams, Jr,
Ashar P Parikh,
Ashley Polizzotto,
Asia D Clark,
Atul J Agrawal,
Barbara J Maulfair,
Barbara Janine Breithaupt,
Bernardica T Sculac Stern,
Betty M Ng Lee,
Bijoy Panicker,
Bo Chi, PhD,
Brandi L Garbutt,
Brandon C Heitmeier,
Brandon L Mariner,
Brandy N Lepage,
Brian M Brodman,
Brooke K Higgins,
Bryan A Galvez,
Burnell M Henry,
Byungja E Marciante,
Carl A Huffman, III,
Carl Lee,
Carrie E Jolly,
Caryn M Mcnab,
Catherine J Laufmann,
Catherine M Beer,
CDR Ileana Barreto Pettit,
CDR Rochelle B Young, RPh, MSA,
Charanjeet Jassal,
Charisse K Green,
Charles D Brown,
Charles M Edwards,
Christian Parra,
Christina A Miller,
Christine M Rivera,
Christopher S Fields,
Christopher S Keating,
Christopher T Middendorf,
Chryste D Best,
Clinton J Lott,
Crystal Monroy,
Cynthia J Lee, MS,
Damaris Y Hernandez,
Dandan Wang, PhD,
Darla J Christopher,
Darren S Brown,
Dave M Deroche,
David M Beltran,
David R Delucia,
Demitria J Argiropoulos,
Demitria J Xiradakis,
Denise M Digiulio,
Dennis Cantellops Paite,
Devon O Seymour,
Deyaa Shaheen,
Dipesh K Shah,
Dolores Harper,
Donald G Gordon,
Donald L Lech,
Dongping Dai, PhD,
Douglas C Kovacs,
Douglas G Ng,
Dr. Barbara D Paul, PhD,
Dr. Robert C Horan, MD,
Duo D Chen,
Edwin Melendez,
Elizabeth B Griffin,
Emest F Bizjak,
Emilio Z Azukwu,
Eric M Mueller, PharmD,
Erica B Brush,
Erica B Raphael,
Erika V Butler,
Evelyn Taha,
Farhana Khan,
Feiyan Jin,
Felix Maldonado,
Francis A Guidry,
Frank Verni,
Frank Wackes,
Frederick F Razzaghi,
Gabriel Feng,
Gajendiran Mahadevan, PhD,
Gam S Zamil,
Gary J Lehr,
Gary T Greco,
Gayle S Lawson,
George J Flynn,
Gerald B Seaborn, Jr,
Geraldine D Sanders,
German Rivera,
Gifford Whitehurst, Jr,
Gregson A Joseph,
Guerlain Ulysse,
Gwyn G Dickinson,
Hala L Selby,
Heika R Tait,
Helen B Ricalde,
Heriberto Negron Rivera,
Iraida Ortiz,
Iram R Hassan, PhD,
J'maica J Hunter,
Jacqueline S Warner,
James A Liubicich,
James C Maclaughlin,
James K Rice,
James L Dunnie, Jr,
James R Fleckenstein,
James S Cartwright,
Jane C Chen,
Jared P Stevens,
Jason P Aun,
Javier O Vega,
Jay T Wong,
Jeffrey A Sommers,
Jeffrey S Buckser,
Jennifer D Hollstrom,
Jennifer J Stone,
Jennifer L Custodio,
Jennifer L Huntington,
Joanne E King,
Joel D Hustedt,
Joel R Powers,
Joey V Quitania,
Jogy George,
John D Mcintosh,
John E Aghadia,
Johnna L Bleem,
Jorge L Gonzalez,
Jorge L Guadalupe,
Jose A Lopez,
Jose Acruz Gonzalez,
José E Meléndez,
Jose M Cayuela,
Jose Ohernandez Guzman,
Jose R Hernandez,
Jose V Obando,
Joseph J Vannelli,
Joseph Maselli,
Joseph Milcetic,
Josh Renzo N Ramilo,
Joy P Matthias,
Joyce A Williams,
Juan Rjimenez Garcia,
Judy Keihl,
Juliana Falla,
Junho Pak,
Justin A Boyd,
Kara D Dobbin,
Karen L Kosar,
Karen Takahashi,
Katherine Szestypalow,
Kathleen D Culver,
Kathy E Craven,
Kathy E Noe,
Kejun Cheng,
Kellia N Hicks,
Kelvin Cheung,
Kenneth H Williams,
Kenneth M Gordon,
Kerry D Noto,
Kerun Hardeo,
Kevin A Gonzalez,
Kevin D Kallander,
Kevin P Foley,
Kham Phommachanh,
Kimberley A Gordon,
Kimberley A Ricketts,
Kimberly A Coward,
Kwong P Lee,
L Gnnmassimia Massimilla, PharmD,
Lance Mde Souza, MBA,
Lareese K Thomas,
Larry K Austin,
Lata C Mathew, PhD,
Latorie S Jones,
Laura Fontan, MS,
Laurimer Kuilan Torres,
Lawrence W Farina,
LCDR Chad N Thompson,
LCDR Michael H Tollon,
LCDR Wilfred A Darang,
Leonard H Lavi,
Lewis K Antwi,
Liming Zhang,
Linda F Murphy,
Lindsey M Schwierjohann,
Lirissia Mccoy,
Lisa M Bellows,
Lisa Mathew,
Loretta J Miller,
Lorie S Hannappel,
LT Tamara J Henderson,
LTJG Matthew R Palo,
Luis A Dasta,
Manhar R Patel,
Marcellinus D Dordunoo,
Marcus F Yambot,
Margaret E Sarles,
Margaret L Bolte,
Margaret M Annes,
Margarita Santiago,
Marian E Ramirez,
Marie A Fadden,
Marie B Buen Bigornia,
Marie Elena C Puleo,
Marie F Morin,
Marion Michaelis,
Mary Jeanet Mcgarry,
Mary M Finn,
Massoud Motamed,
Matthew A Spataro,
Megan A Haggerty,
Meisha A Mandal,
Meisha R Sampson,
Meisha Waters,
Melissa A Henaghan,
Melissa J Garcia,
Melissa N Gregorio,
Mercedes G Castillo,
Michael A Charles,
Michael A Taylor,
Michael E Maselli,
Michael G Mayfield,
Michael G Sinkevich,
Michael K Larson,
Michael L Chasey,
Michael P Sheehan,
Michael R Dominick,
Michael R Goga,
Michael S Araneta,
Michael Serrano,
Michele Gottshall,
Michele L Obert,
Michele Perry Williams,
Miguel A Martinez Perez,
Milan S Mcgorty,
Mindy M Chou,
Morgan J Matthews,
Mra B Kamberem,
Mra Davism,
Mra Mcculloughj,
Mra Munizn,
Mra V Millarw,
Muralidhara B Gavini, PhD,
Nadeem I Chaudhry,
Nancy A Saxenian Emmons,
Nancy G Schmidt,
Nancy L Neiger,
Nazmul Hassan,
Nebil A Oumer,
Neil J Bonzagni, PhD MPH,
Nerizza B Guerin,
Nibin Varghese, MS,
Nicholas C Mendiola,
Nicholas L Paulin,
Nicole Vaught,
Noreen Muñiz,
Nur M Faisal,
Omotunde O Osunsanmi,
Paraluman S Leonin,
Parul M Patel,
Patricia D Stahnke,
Patrick C Klotzbuecher,
Patrick L Wisor,
Paul A Bonneau,
Paul E Stein,
Paul L Bellamy,
Paul M Kawamoto,
Paul Mouris,
Perez,
Peter Abel,
Peter M Trunk,
Peter R Caparelli,
Philip F Istafanos, DMV, MS,
Pratik S Upadhyay, DDC,
Rachael O Oyewole,
Rachel C Harrington,
Rafael E Arroyo,
Rajiv R Srivastava,
Rakhi B Shah,
Ralph H Vocque,
Ramon E Martinez,
Raymond M Lam,
Raymond T Oji,
Reba A Gates,
Rebecca E Dombrowski,
Rebecca Parrilla,
Regina T Brown,
Richard H Penta,
Rita F Monfort,
Rita K Kabaso,
Robert C Coleman,
Robert C Howard,
Robert C Steyert,
Robert D Tollefsen,
Robert E Moncayo,
Robert J Doyle,
Robert J Martin,
Robert M Barbosa,
Robin P Mathew,
Rochelle K Kimmel,
Rochelle L Cross,
Rodney G Raiford,
Ronald Ifraimov,
Rose Xu,
Ruben C Ayala, PharmD,
Ruben C Quintana,
Rumany C Penn, PharmD,
Russell J Glapion,
Russell K Riley,
S Lori Brown, PhD MPH,
Sachinkumar V Patel,
Saied A Asbagh,
Saleem A Akhtar,
Samina S Khan,
Samuel K Mathew,
Samuel T Walker,
Sandra A Hughes,
Sarah A Meehan,
Sarah A Wangseng,
Sarah E Mcmullen,
Sarah E Rhoades,
Sateesh Kum Sathigari,
Saundrea A Munroe,
Scott R Izyk,
Scott T Ballard,
Sean R Marcsisin,
Sharon K Thoma, PharmD,
Shelby N Turner,
Shirley J Berryman,
Shreya Shah,
Simone E Pitts,
Sina Shojaee,
Sixto M Mercado Rios,
Sneha S Patel,
Sony Mathews,
Sonya L Karsik, RAC,
Srinivas R Chennamaneni, PhD,
Stacey S Degarmo,
Stacie A Woods,
Stephanie A Slater, MS,
Stephanie T Durso,
Stephen D Brown,
Stephen J Koniers,
Stephenie M Ortiz,
Steven A Gonzales,
Steven C Madzo,
Steven Frisbee,
Steven M Weinman,
Steven P Donald,
Sunita Vij,
Susan M Jackson,
Susan M Joseph,
Susan P Bruederle,
Susan T Hadman,
Susanna E Ford,
Suzanne N Vallez,
Syeda N Mahazabin,
Tamil Arasu, PhD,
Tanya R Syffrard,
Tara G Bizjak,
Tara K Normandin,
Tara L Greene,
Tekalign Wondimu,
Terry Bridgewater,
Terry E Pierre,
Thomas J Arista,
Thomas J Mooney,
Thomas W Gordon,
Tina M Pawlowski,
Tomika L Bivens,
Tonia F Bernard,
Tonya R Johnson,
Toyin B Oladimeji,
Truong Xuan Nguyen (Andy),
Tyanna N Hadley,
Uduak M Inokon,
Unnee Ranjan,
Uttaniti Limchumroon (Tom),
Valerie A Potopsingh,
Valerie L Whipp,
Vanessa M Williams,
Vickie J Kanion,
Vilmary Negron Rodriguez,
Viviana Matta,
Vivin George,
Vlada Matusovsky,
Walden H Lee,
Wayne D Mcgrath,
Wayne E Seifert,
Wayne S Thompson,
William A Warnick,
William J Leonard,
Winston R Alejo,
Xiaokuang Lai, PhD,
Xiaoping Guan,
Xiomara Copeland,
Yasmin B Rios,
Youmin Wang,
Yumi J Hiramine,
Yuyan Liang,
Yvesna C Blaise,
Yvins Dezan,
Yvonne M Santiago,
Zhong Li, PhD,
Zhongren Wu
Satheesh Thomas's Documents
Experience Redica Systems’ NEW investigator profiles and dashboards
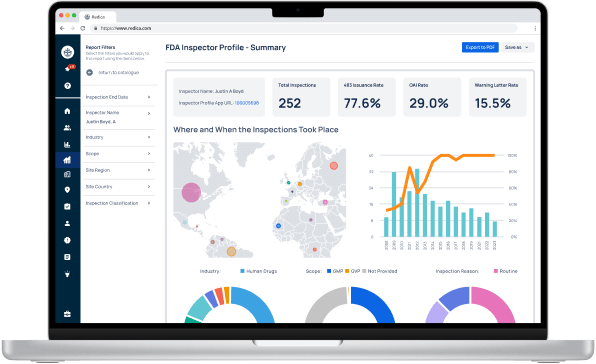
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more