FDA Investigator Sharon Giamberini
Sharon Giamberini has conducted inspections on 147 sites in 3 countries as of 05 Jun 2024. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
147
Last Inspection Date:
05 Jun 2024
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
Norway,
Poland
FDA Investigators that have inspected at least one site in common with Sharon Giamberini:
Aaron F Coleman,
Aaron J Fox,
Abraham M Maekele,
Albert A Salvador,
Alec D Fabus,
Alexander B Anderson,
Alysia C Alger,
Ana Delp Cintron,
Ana P Barido,
Anabel Veiga,
Andrea D Charles Julien,
Andrew J Mcguffin,
Angela E Glenn,
Angela F Orozco,
Anthony W Lee,
Ashley A Mutawakkil,
Ashley A Segura,
Ashley B Jelonek,
Ashley Gonzalez,
Benita C Okeke,
Betsy C Galliher,
Billy M Battles,
Brant M Schroeder,
Brian M Carril,
Bruce Quijano,
Carla A Norris,
Carlos H Ruiz,
Carol S Davis,
CDR Ileana Barreto Pettit,
CDR Sean T Creighton,
Celia L Hutchinstein,
Charles S Watson,
Christopher T Smith,
Chyla T Hunter,
Cindy Laboy,
Clara E Santiago,
Colleen M Aspinwall,
Courtney A Gilbert,
Craig A Garmendia,
David M Pearson,
David P King,
Delkis J Caycedo,
Dillard H Woody, Jr,
Donald J Mee,
Dorothy P Kramer,
Eduardo Carbonell,
Edward A Fabiano,
Edwin J Gorney,
Emily A Baldwin,
Emily A Green,
Eric R Holm,
Ernest A Clausnitzer,
Ernest L Levins,
Ethan P Stegman,
Frances M Rivera,
Frances Mrivera Rosario,
Gabriel E Muniz,
Gene R Gunn,
Gerard D Difiore,
Gina C Chen,
Gina Eng,
Giovanna Serpa,
Glenn S Quintanilla,
Haijing Hu,
Harry R Bringger, Jr,
Hector Vallellanes,
Humphrey,
Ivis L Negron,
Jacqueline E Manjone,
James To Neal,
Jason D Tenney,
Jason P Aun,
Jeffrey G Baravik,
Jennifer A Robinson,
Jennifer Caycedo,
Jennifer L Huntington,
Jennifer Lalama,
Jennifer M Menendez,
Jessica L Pressley,
Joanne E King,
John J Bernal,
Jontae D Sanders,
Joseph P Dudley,
Juan C Gonzalez Rivera,
Karen M Rodriguez,
Karissa J Mccaw,
Karl D Hezel,
Karlton T Watson,
Katherine M Taylor,
Kelly M Byers,
Laura M Lopez,
Lenora M Amason,
Leo J Lagrotte,
Leroy Terrelonge,
Leslin M Coachman,
Leyden Guerrero,
Linda K Matheny,
Lisa A Warner,
Lissette Paiz,
Lizaida E Rodriguez,
Lourdes Andujar,
LT Daveta L Bailey,
LT Kimberly M Hull,
LT Prince D Brown,
LT Tamara J Henderson,
Luis A Martinez Larracuente,
Lundy H Patrick,
Luz I Collado,
Marc Balzarini,
Marinee Flores Marrero,
Mary F Bodick,
Matthew B Thomaston,
Megan K Otero,
Melissa J Hill,
Mendoza,
Meredith M Cobb,
Meredith P Soehl, Investigator,
Michael C Lombardi,
Minerva Rogers,
Neimar Lopez,
Nicole E Knowlton,
Nicole M Bell,
Norton,
Omayra Nrodriguez Ruiz,
Ormond,
Pablo Alcantara,
Pablo Feliciano,
Paul A Licata,
Paul H Dakin,
Rachel A Gomez,
Rachel A Peters,
Raymond A Lyn, Jr,
Reynolds,
Rina Bhikha,
Robert J Goldman,
Rodney W Lenger,
Ronan F King,
Roy R Rinc,
Rozelle G Smith,
Sally Gopaul,
Sandra Carpio,
Saundrea A Munroe,
Sheila P Barthelemy,
Sherbet L Samuels,
Sheri L Stephenson,
Sonia M Monges,
Stacey A Meyer,
Stacey F Allard,
Stacey L Witherwax,
Stephanie C Milan,
Stephanie D Crockett,
Susan M Turcovski,
Teresa I Navas,
Teresa M Hillebrandt,
Timothy A Tennell,
Toby L Keaton,
Tracy L Reed,
Victor Spanioli,
William A Oetting,
Yazmith Guzman
Sharon Giamberini's Documents
| Publish Date | Document Type | Title |
|---|---|---|
| January, 2018 | FDA 483 Response | OXYGEN DEVELOPMENT, L.L.C. - Form 483R, 2018-02-01 |
| September, 2021 | EIR | Natures Health Options, LLC - EIR, 2021-09-28 |
| September, 2021 | EIR | Be Powerful, LLC - EIR, 2021-09-07 |
| January, 2018 | FDA 483 | OXYGEN DEVELOPMENT, L.L.C. - Form 483, 2018-01-17 |
| September, 2021 | FDA 483 | Natures Health Options, LLC - Form 483, 2021-09-28 |
| January, 2018 | EIR | OXYGEN DEVELOPMENT, L.L.C. - EIR, 2018-01-19 |
| January, 2018 | EIR | OXYGEN DEVELOPMENT, L.L.C. - EIR, 2018-01-17 |
| August, 2016 | FDA 483 | Florida Crystals Food Corporation - Form 483, 2016-08-11 |
| September, 2021 | FDA 483 | Be Powerful, LLC - Form 483, 2021-09-07 |
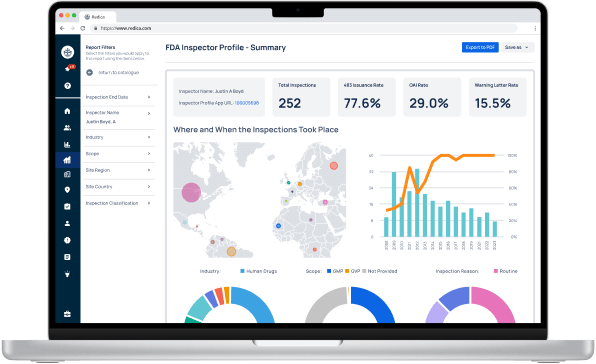
Experience Redica Systems’ NEW investigator profiles and dashboards
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more