FDA Investigator Thomas R Withers
Thomas R Withers has conducted inspections on 105 sites in 2 countries as of 10 Jun 2024. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
105
Last Inspection Date:
10 Jun 2024
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
Fiji
FDA Investigators that have inspected at least one site in common with Thomas R Withers:
Adam R Cooke,
Addie V Scott Boston,
Adriana N Jimenez Lopez,
Albert A Salvador,
Ali Akbar,
Amanda B Athey,
Amanda M Osteen,
Amber G Chung,
Amos C Epps,
Amy H Ruble,
Amy N Chen,
Anastasia M Shields,
Andrea L Williams Jones,
Andrew J Howard,
Andrew W Harmon,
Angel K Sandhu,
Angela J Alexander,
Angela Jourdain,
Anh M Lac,
Ann Marie Montemurro,
Anthony F Lorenzo,
Asamoah Asamoah,
Ashar P Parikh,
Austin B Appler,
Barbara M Frazier,
Ben Du,
Billy M Battles,
Brandi L Garbutt,
Brandon J Brookens,
Brandon L Mariner,
Brandon M Kurjanowicz,
Brandy N Lepage,
Brian L Belz,
Brian M Palermo,
Brian R Yaun,
Brooke K Higgins,
Brooke K Seeman,
Bruce E Kummer,
Bryan J Love,
Burnell M Henry,
Calvin W Edwards,
Candace Y Gomez Broughton,
Candice C Mandera,
Candice J Cortes,
Carl E Lovrich,
Carl Lee,
Carlos M Gonzalez,
Carolyn A Renshaw,
Carrie L Doupnik,
Catherine V Quinlan,
CDR Ileana Barreto Pettit,
CDR Rochelle B Young, RPh, MSA,
CDR Thomas R Berry, PPh,
Charlean R Butler,
Charles E Idjagboro,
Cherrie A Zachary,
Christian D Lynch (CDL),
Christina K Theodorou,
Christina Theodorou,
Christina V Santos,
Claire M Minden,
Cody D Rickman,
Collins,
Craig D Zagata,
Cynthia A Harris, MD, RN,
Cynthia Jim, CSO,
Cynthia White,
Daniel J Mechenbier,
Daniel R Tammariello,
Daniel T Lee,
David A Oluwo,
David J Leray,
David P Rice, Jr,
Debra L Pagano,
Dennis L Doupnik,
Dennis R Hock,
Devaughn Edwards,
Dianne H Milazzo,
Donald C Obenhuber, PhD,
Douglas A Campbell,
Dr. Gang Wang, PhD,
Dr. Robert C Horan, MD,
Dr. Zhou Chen (nmi), MD PhD,
Dung N Tran,
Dustin P Tran,
Edward P Potter,
Eileen A Liu,
Emest F Bizjak,
Emmanuel Adu Gyamfi, PhD,
Eric L Dong, BS,
Erica L Nicoll,
Erik T Nagaoka,
Erika M Wilkerson,
Erin C Hill,
Erin Hill (EH),
Erma Zaimova,
Esaw,
Fabian Nchaparro Rodriguez,
Gabriel R Mclemore,
Gale L Glinecki,
Gayle S Lawson,
George Pyramides,
Gerald B Seaborn, Jr,
Gideon N Esuzor,
Gifford Whitehurst, Jr,
Gladys B Casper,
Gregory E Beichner,
Hala L Selby,
Heath A Gerber,
Heath R Harley,
Hector A Carrero,
Helen B Ricalde,
Henry K Lau,
Hung H Do, MS,
Iqra Iftikhar,
Jacqueline Johnson,
Jacqueline Mdiaz Albertini,
James M Odonnell,
James M Simpson,
James W Leonette,
Jamie D Richardson,
Jamie D Swann,
Jared P Stevens,
Jason F Chancey,
Jawaid Hamid,
Jay Slater, MD,
Jefferson,
Jeffrey M Watson,
Jennifer D Hollstrom,
Jennifer L Bridgewater,
Jessica D Weber,
Jessica L Pressley,
Joey C West,
Jogy George,
John Dan,
John M Gusto,
Johnson,
Jolanna A Norton,
Jonah S Ufferfilge,
Jonathan W Chapman,
Jose R Hernandez,
Joseph F Owens,
Joslyn K Brunelle, PhD,
Judith A Paterson,
Julianne C Mccullough,
Junho Pak,
Justin A Boyd,
Justin A Hefferman,
Karen A Spencer,
Katelyn Astaub Zamperini,
Kathleen D Culver,
Kenneth L Smalls,
Kenneth Nieves,
Kevin P Foley,
Kristina L Conroy,
Kula N Jha,
L'oreal F Walker,
Lance Mde Souza, MBA,
Larry K Hampton,
Laura Fontan, MS,
Laurel A Beer,
Lawrence Carter, III,
Lawrence Harmon, Jr,
Lawrence J Stringer,
LCDR Debra Emerson,
LCDR Matthew R Mcnew, MPH, REHS/RS,
LCDR William H Bender,
Lillian C Hsu,
LTJG Bradley E Benasutti,
Luis A Dasta,
Marcelo O Mangalindan, Jr,
Marcus A Ray,
Marie F Morin,
Marjorie L Davis,
Martha Sullivan Myrick,
Mary R Kirker,
Matthew J Gretkierewicz,
Maureen A Wentzel,
Melanie M Walker,
Melissa J Garcia,
Michael D Omeara,
Michele L Forster, PhD,
Michelle Yclark Stuart,
Mihaly S Ligmond,
Monica Cburgos Garcia,
Murthy Vkn Sikha,
Nash,
Natasha A Dezinna,
Nawab A Siddiqui,
Neali H Lucas,
Nebil A Oumer,
Nisha C Patel,
Omotunde O Osunsanmi,
Parul M Patel,
Paul L Bellamy,
Paula A Trost,
Paula J Bretz,
Penny H Mccarver,
Perry H Gambrell,
Philip W Beck,
Prabhu P Raju,
Qin Xu,
Rachel C Harrington,
Randy L Clarida,
Raymond T Oji,
Raymond W Niles,
Renisha M Shaw,
Rhonda Alexander,
Robert C Coleman,
Robert J Maffei,
Robin Levis, PhD,
Rose Ashley,
Saleem A Akhtar,
Sam Pepe,
Samuel M Aboagye,
Sarah J Park,
Saundrea A Munroe,
Seneca D Toms,
Shafiq S Ahadi,
Sherri J Jackson,
Shusheen A Alexander,
Sidney B Priesmeyer,
Sierra M Shockley,
Simone E Pitts,
Slatten,
Stephen C Eason,
Stephen D Brown,
Steven J Thurber,
Steven L Carter,
Steven P Donald,
Susan M Jackson,
Swati Verma,
Tamara N Champion,
Tamika D Cathey,
Tammara A Stephens,
Tammy M Phillips,
Tania E Vizcaino,
Temar Q Williams,
Thai D Truong,
Thomas J Arista,
Tiki J Dixon,
Toyin B Oladimeji,
Travis R Hunt,
Tressa T Lewis,
Uduak M Inokon,
Uttaniti Limchumroon (Tom),
Van Cliff,
Vesna V Stanoyevitch,
Vicky C Stoakes,
Viviana Matta,
Vlada Matusovsky,
Wayne E Seifert,
Wayne L Jefferson,
Wayne T Smith,
William A Warnick,
William D Bassett, Jr,
William D Murray,
William J Leonard,
William V Millar,
Ying Xin Fan, PhD,
Yiyue Zhang (nmi), PhD,
Yun Wu, PhD,
Zhong Li, PhD,
Zhongren Wu
Thomas R Withers's Documents
| Publish Date | Document Type | Title |
|---|---|---|
| October, 2023 | FDA 483 | Afton Scientific, LLC - Form 483, 2023-10-06 |
| October, 2022 | EIR | PACKAGING COORDINATORS LLC - EIR, 2022-10-21 |
| December, 2022 | FDA 483 | BioMarin Pharmaceutical Inc. - Form 483, 2022-12-09 |
| October, 2022 | FDA 483 Response | PACKAGING COORDINATORS LLC - Form 483R, 2022-11-03 |
| November, 2022 | FDA 483 | Barr Laboratories Inc. - Form 483, 2022-11-04 |
| August, 2022 | EIR | Patheon Manufacturing Services LLC - EIR, 2022-08-04 |
| March, 2023 | FDA 483 | Alk-abello, Inc. - Form 483, 2023-03-09 |
| August, 2022 | FDA 483 | Patheon Manufacturing Services LLC - Form 483, 2022-08-04 |
| August, 2022 | FDA 483 Response | Patheon Manufacturing Services LLC - Form 483R, 2022-08-25 |
| October, 2022 | FDA 483 | PACKAGING COORDINATORS LLC - Form 483, 2022-10-21 |
Experience Redica Systems’ NEW investigator profiles and dashboards
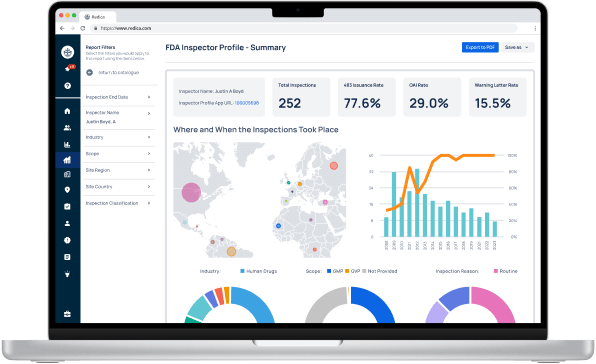
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more