FDA Investigator Craig A Garmendia
Craig A Garmendia has conducted inspections on 217 sites in 29 countries as of 29 Apr 2024. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
217
Last Inspection Date:
29 Apr 2024
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
India,
France,
Australia,
New Zealand,
Canada,
Belize,
Thailand,
Moldova (Republic of),
Germany,
Spain,
Austria,
Poland,
Hungary,
United Kingdom of Great Britain and Northern Ireland,
Korea (Republic of),
Japan,
South Africa,
Italy,
Switzerland,
Argentina,
Czechia,
Ukraine,
Georgia,
Denmark,
Puerto Rico,
Taiwan,
China,
Norway
FDA Investigators that have inspected at least one site in common with Craig A Garmendia:
Aaron F Coleman,
Adam M Derr,
Ademola O Daramola,
Alanna L Mussawwir Bias,
Albert A Salvador,
Allen F Hall,
Amanda E Lewin, PhD,
Ana Delp Cintron,
Ana P Barido,
Andrea A Branche,
Anita Narula, PhD,
Annette Melendez,
Anthony J Grace,
Arindam Dasgupta, PhD,
Arsen Karapetyan,
Barbara A Rusin,
Barbara D Wright,
Barbara M Frazier,
Benita C Okeke,
Betsy C Galliher,
Bill Tacket, Jr,
Bonita S Chester,
Brooke K Higgins,
Brunilda Torres,
Byungja E Marciante,
Cara L Alfaro,
CDR Ileana Barreto Pettit,
CDR Rochelle B Young, RPh, MSA,
Cheryl A Grandinetti,
Chiaochun J Wang,
Chikako Torigoe, PhD,
Christian D Lynch (CDL),
Christos G Tsingelis,
Clara E Santiago,
Clotia Cabbey Mensah,
Colleen M Aspinwall,
Courtney A Gilbert,
Courtney A Hunt,
Courtney N Long,
Craig T Rybus,
Dandan Wang, PhD,
Daniel J Roberts,
Daniel L Aisen,
David R Heiar,
Dawn C Olenjack,
Dawn M Mccabe,
Dawne M Hines,
Dennis R Hock,
Devaughn Edwards,
Dianiris C Ayala,
Dianne H Milazzo,
Don A Leeseberg,
Dr. Gopa Biswas, PhD,
Dr. Sriram Subramaniam, PhD,
Dyvette Arline,
Eduardo Carbonell,
Edwin J Gorney,
Emily A Baldwin,
Emir Galevi,
Ethan P Stegman,
Fabian Nchaparro Rodriguez,
Farhana Khan,
Figarole,
Gabriel E Muniz,
Gajendiran Mahadevan, PhD,
Gene R Gunn,
George C Amedro,
George Pyramides,
Gerald N Mcgirl, DDS,
German Rivera,
Gisselle I Sensebe,
Gloria E Parra,
Gulshan R Anand,
Habacuc V Barrera,
Hasan A Irier, PhD,
Heather M Bullock,
Hector Jcolon Torres,
Himanshu Gupta, PhD,
Holly Brevig,
Jacqueline Mdiaz Albertini,
James E Frye,
James L Dunnie, Jr,
James M Kewley,
James P Mcevoy,
James To Neal,
Jana L Caylor,
Janete F Guardia,
Jean M Mulinde, MD,
Jeanne J Thai,
Jennifer Adams, MPH,
Jennifer C Adams,
Jennifer D Hollstrom,
Jennifer L Huntington,
Jennifer L Sheehan,
Jennifer Lalama,
Jennifer M Menendez,
Jessica L Pressley,
Joann Walters Salvatierra,
Joanne E King,
Jogy George,
John A Kadavil, PhD,
Jonee J Mearns,
Jose Acruz Gonzalez,
Ka L Wong,
Kara A Scheibner, PhD,
Karen M Cooper,
Karen M Rodriguez,
Karl D Hezel,
Katherine M Taylor,
Kathleen V Ferguson,
Kathryn A Krentz,
Kelly M Byers,
Ladislav Kermet,
Lakecha N Lewis,
Larry K Austin,
Laura E Garcia,
Lawrence Y Lee, PhD,
LCDR Angelica M Chica,
LCDR Randall L Morris,
Leo J Lagrotte,
Leslie A Jackanicz,
Li Hongpaul Yeh, PhD,
Libia M Lugo,
Liming Zhang,
Linda K Matheny,
Linda R Kuchenthal,
Lisa P Oakes,
Lori A Gioia,
Lt Bill Tackett, Jr,
LT Richard A Lyght,
Luz I Collado,
Makini Cobourne Duval, PhD,
Marc Balzarini,
Margaret Torres Vazquez,
Maria V Price,
Mark V Preciados,
Martin K Yau, PhD,
Matthew C Watson,
Matthew F Duff,
Matthew T Sanchez,
Melissa D Ray,
Melkamu Getie Kebtie, PhD,
Mendoza,
Michael A Charles,
Michael C Lombardi,
Michael E Maselli,
Michael P Anthony,
Michael Serrano,
Michelle D Haamid,
Michelle M Noe Varga,
Michelle Yclark Stuart,
Miguel A Martinez Perez,
Minh D Phan,
Mizanne E Lewis,
Mohsen Rajabi Abhari, FDA,
Mra Mcculloughj,
Nancy A Bellamy,
Nicola M Fenty Stewart,
Nicolas Riveratorres,
Nicole E Knowlton,
Nicole M Bell,
Niketa Patel,
Nilufer M Tampal, PhD,
Omayra Nrodriguez Ruiz,
Ormond,
Parul M Patel,
Patricia F Hughes, PhD,
Patrick L Wisor,
Paul L Figarole, Jr,
Paula M Laplant,
Perry H Gambrell,
Prabhu P Raju,
Rachel A Peters,
Rachel C Dailey,
Ramon H Pabon Aponte,
Randy L Clarida,
Raymond A Lyn, Jr,
Rebecca E Dombrowski,
Rebecca Rodriguez,
Rebecca T Davis,
Reyes Candau Chacon, PhD,
Richard K Vogel,
Richard W Berning,
Rina Bhikha,
Robert D Tollefsen,
Ronald Ifraimov,
Roy R Rinc,
Ruben C Ayala, PharmD,
Ruth A Williams,
Samantha J Pinizzotto, D V M,
Samina S Khan,
Sandra S Saniga,
Sarah E Mcmullen,
Saundrea A Munroe,
Scott B Laufenberg,
Seongeun Cho (Julia), PhD,
Sereen Gmorgan Murray,
Sharon Giamberini,
Sharon L Matson,
Sherbet L Samuels,
Sheri L Stephenson,
Sidney B Priesmeyer,
Sonia M Monges,
Sripal R Mada, PhD,
Stacey L Witherwax,
Stanley B Eugene, BS, BME,
Stephanie D Crockett,
Stuart W Russell,
Sunitha K Rajaram, PhD,
Susan D Yuscius,
Susan M Corrales,
Susan M Turcovski,
Suzanne N Vallez,
Tawny L Colling,
Teresa I Navas,
Thea C Grome,
Thomas J Arista,
Thomas W Nojek,
Thuy T Nguyen, LCDR,
Timothy C Grome,
Tina A Desorcy,
Tina M Pawlowski,
Torrance J Slayton,
Tracey L Harris,
Traci M Armand,
Vanessa E Coulter,
Victor Spanioli,
Virginia L Meeks,
Viviana Matta,
Walden H Lee,
Wayne E Seifert,
William P Tonkins,
Xianghong Jing (Emily), PhD,
Xikui Chen (nmi), PhD,
Yiyue Zhang (nmi), PhD,
Young M Choi, PhD,
Yumi J Hiramine,
Zerita White
Craig A Garmendia's Documents
Experience Redica Systems’ NEW investigator profiles and dashboards
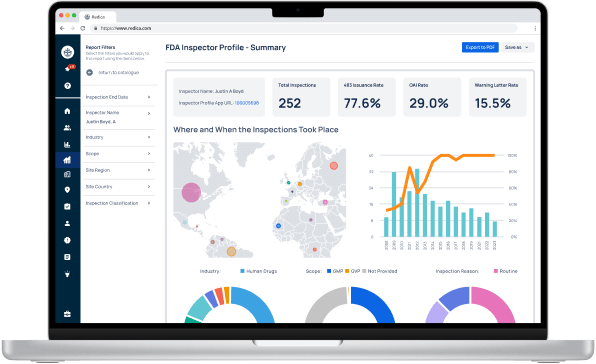
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more