FDA Investigator Stephanie A Slater, MS
Stephanie A Slater, MS has conducted inspections on 122 sites in 10 countries as of 24 Mar 2021. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
122
Last Inspection Date:
24 Mar 2021
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
Germany,
China,
France,
Italy,
Canada,
Ireland,
India,
Korea (Republic of),
Japan
FDA Investigators that have inspected at least one site in common with Stephanie A Slater, MS:
Aaron J Adler,
Aaron J Poloni,
Abby M Miller,
Alan D Grupe,
Alan P Kurtzberg,
Alicia Gilbert,
Amalia C Himaya,
Amanda L Fyles,
Amir Alavi,
Anderson,
Andrea A Branche,
Andrea George, PhD,
Andrea P Scott,
Andrei Perlloni,
Andrew C Chang,
Andrew K Haack, PhD,
Andy B Lee,
Angel K Sandhu,
Angela D Wise,
Anh M Lac,
Anita Narula, PhD,
Ann Marie Montemurro,
Ann Marie Schofield,
Anna Lazar,
Anthony C Warchut,
Anthony E Keller, RPh,
Arsen Karapetyan,
Ashar P Parikh,
Ashley N Queen, PhD,
Azza Talaat,
Babajide Michael Osunsanmi,
Barbara J Rincon,
Barbara J Wilcox, PhD,
Benigno B Devera,
Bijoy Panicker,
Binh T Nguyen,
Bo Chi, PhD,
Brenda K Alford,
Brian J Ryan,
Brien C Fox,
Bruce R Burrell,
Bryan A Galvez,
Bryan S Roddy,
Bryce E Mansfield,
Bush,
Calvin B Koerner,
Capri R Woolridge,
Carl Lee,
Carla J Lundi,
Carole L Jones,
Carolyn A Renshaw,
Caryn M Everly,
Caryn M Mcnab,
Catherine J Laufmann,
Cathleen A Carr Sharpe,
CDR Ileana Barreto Pettit,
CDR Rochelle B Young, RPh, MSA,
Charisse K Green,
Charles I Ann,
Chelsea N Sealey,
Christian D Lynch (CDL),
Christopher D Washington,
Christopher M Jenner,
Christopher R Czajka,
Christopher T Middendorf,
Claudia Mperez Kasmarski,
Comyar Shoghi,
Craig K Hopkins,
Creighton T Tuzon,
Ct Viswanathan, PhD,
Cynthia J Lee, MS,
Cynthia Jim, CSO,
Cynthia L Gorveatt,
Dandan Wang, PhD,
Daniel J Lahar,
Daniel J Roberts,
Daniel Lagasse,
Daniel R Solis,
Darren S Brown,
David G Eng,
David Serrano,
Debra I Love,
Dennis R Downer,
Desiree C Iya,
Deyaa Shaheen,
Diana M Rand,
Diane Cvan Leeuwen,
Dianne B Latona,
Dipti Kalra,
Dogbeda F Mackenzie,
Donna Mwilliams Hill, PhD,
Dr. Chunchang Fang,
Dr. Mark J Seaton, PhD,
Dr. Ralph M Bernstein, PhD,
Dr. Robert C Horan, MD,
Durell L Giles,
Dustin P Tran,
Dustin R Abaonza,
Eileen A Liu,
Elizabeth Juarez,
Elmina E Akwo,
Emily Shacter,
Eric L Dong, BS,
Eric L Scott,
Erica R Mahone,
Esther B Gamallo Herrera,
Evelyn Wong,
Farhana Khan,
Felix Maldonado,
Francis A Guidry,
Gary R Wong,
Gene D Arcy,
Gerald Feldman,
Gerald M Feldman, PhD,
Gerard Pde Leon,
Gerardo Z Vazquez,
Haley H Seymour,
Heidy C Perales,
Heika R Tait,
Helen B Ricalde,
Helen Verdel,
Henry K Lau,
Hugh A Grimoldby,
Ivonne A Vicente,
Jacqueline Mdiaz Albertini,
Jai P Singh,
James A Lane,
James C Lee, PharmD,
James C Maclaughlin,
James E Trabert,
James R Evans,
Jane S Wernberg,
Jannifer L Degroot,
Jazmine M Welcher,
Jean Brewer,
Jean R Mccurry,
Jeanne Fringer, PhD,
Jeanne M Weishaar,
Jeffrey M Watson,
Jeffrey P Raimondi,
Jennifer L Johnson,
Jennifer M Gogley,
Jessica B Clark,
Jessica L Pressley,
Jiao Yang, PhD,
Jinkee Mvila Binayug,
Jinnie Kokiatkulkij,
Joan T Briones,
Joanne Hosting,
Jocelyn E Massey,
Jocelyn E Sparks,
Joel D Hustedt,
Joey V Quitania,
John A Gonzalez,
Jolanna A Norton,
Jonathan T Little,
Jonathan W Chapman,
Jorge L Guadalupe,
Jose Acruz Gonzalez,
José E Meléndez,
Joseph George,
Joseph Kutze, PhD,
Joseph P Iser, MD,
Joshua S Hunt,
Juanita Banuelos,
Judy C Nepsa,
Julian C Hanson,
Julie A Rorberg,
Julie A Stocklin,
Julie D Bringger,
Junho Pak,
Justin A Boyd,
Justine Tomasso,
Karen D Phung,
Karen E D'orazio,
Karen M Montgomery (KMM),
Karen P Langer,
Kari M Johansen,
Katherine E Jacobitz,
Katherine L Arnold,
Kathryn E King,
Kathy Chiu,
Kathy Kuo,
Keeshunna D Daniels,
Keishla M Arroyo Lopez,
Keith O Webber, PhD,
Keith Webber, PhD,
Kelley,
Kelsey A Hustedt,
Kelsey A Volkman,
Kelvin Cheung,
Kelvin X Sanders,
Kenneth H Williams,
Kenneth O Gee, PhD,
Kenneth S Boehnen,
Kevin P Foley,
Kevin T Gerrity,
Kham Phommachanh,
Khoa Nathanv Tran,
Kim Lthomas Cruse,
Kimberly Jproctor Jones,
Kouros Kangarli,
Kristin M Abaonza,
Kumar G Janoria,
Lakecha N Lewis,
Lakisha M Williams,
Lance Mde Souza, MBA,
Lanita F Kelley,
Larry K Austin,
Lauren E Swantko,
Laurie Graham,
Laurie P Norwood,
Laurimer Kuilan Torres,
Lawrence Y Lee, PhD,
LCDR Ismael Olvera, IV,
LCDR Jennifer H Rhyu,
Leon R Marquart,
Leonard H Lavi,
Leya A Grosch,
Lillian S Wu,
Liming Zhang,
Linan Ha, PhD,
Linda F Murphy,
Linda S Leja,
Linda Thai,
Lindsey M Schwierjohann,
Ling Yu L Liu,
Linh Tu,
Lisa Shin,
Lori J Jennings,
Lori J Silverstein,
Lorie S Hannappel,
Louis B Cencetti,
Lucila B Nwatu,
Luella J Rossi,
Luis A Dasta,
Lynda L Perry, PhD,
Mabel M Lee,
Maida Henesian (NMI),
Marcus F Yambot,
Margaret M Annes,
Margo C Jones,
Maria Pkelly Doggett, MBA,
Marie Falcone,
Marie K Kinkade,
Marie T Falcone,
Marijo B Kambere, PhD,
Mariza M Jafary,
Mark A Tucker,
Mark C Saale,
Mark E Imsland,
Marsha L Mccauley,
Marsha W Major,
Marshalette O Edwards,
Mary E Farbman, PhD,
Mary Ewilkerson Brinsko,
Mary R Hole,
Matthew B Casale,
Matthew J Johnson,
Matthew R Clabeaux,
Maurice M Sheehan,
Maxyne T Lam,
Mei Chiung Huang (Jo),
Michael A Charles,
Michael D Garcia,
Michael D Kawalek,
Michael J Donovan,
Michael P Sheehan,
Michael R Goga,
Michael R Klapal,
Michael Shanks, MS,
Michael T Cyrus,
Michele L Obert,
Michelle Rfrazier Jessen, PhD,
Miguel A Martinez Perez,
Mihaly S Ligmond,
Milos Dokmanovic, PhD,
Min Shanmabel Liu,
Misty L Wallace,
Mra B Kamberem,
Mra Davism,
Mra Mcculloughj,
Muralidhara B Gavini, PhD,
Nadeem I Chaudhry,
Nancy E Byerly,
Nancy E Kirschbaum,
Nancy T Waites,
Narahi J Alvarez Alcazar,
Natalia Pripuzova, PhD,
Nayan J Patel,
Nga T Ho,
Nicholas A Violand,
Nicholas L Hunt,
Nicholas Obiri, PhD,
Olumide A Martins,
Omotunde O Osunsanmi,
Parul M Patel,
Patricia F Hughes, PhD,
Patrick C Klotzbuecher,
Patrick E Gainer,
Patsy J Domingo,
Paul F Rader,
Paul M Kawamoto,
Paul Mouris,
Paula A Trost,
Peter E Baker,
Peter Kessler, PhD,
Prabhu P Raju,
Qing Joanna Zhou, PhD,
Rafael A Kaup,
Ralph A Erickson,
Raquel Gonzalez Rivera,
Raymond T Oji,
Reba A Gates,
Rebecca Rodriguez,
Rebecca T Davis,
Reyes Candau Chacon, PhD,
Richard A Martin,
Richard C Chiang,
Richard W Thornton,
Richmond K Yip,
Riley C Myers, PhD,
Robert C Coleman,
Robert D Tollefsen,
Robert J Martin,
Robert Jennings,
Rocco C Black,
Roger F Zabinski,
Roman T Drews, PhD,
Rose Ashley,
Rumany C Penn, PharmD,
Russell J Glapion,
Russell K Riley,
Ruth Moore, PhD,
Ryan J Borges,
S Lori Brown, PhD MPH,
Saied A Asbagh,
Samantha J Bradley,
Samina S Khan,
Samuel J Barone, III,
Sandra S Saniga,
Sangeeta M Khurana, PhD,
Santiago Gallardo Johnson,
Santos E Camara,
Sara H Gabel,
Sarah E Mcmullen,
Sarmistha Sanyal, PhD,
Satheesh Thomas,
Saundrea A Munroe,
Scott N Lim,
Scott T Ballard,
Sean P Desbrow,
Selene T Torres,
Serge L Beaucage,
Shahriar Rahmani,
Shannon A Gregory,
Sharon K Thoma, PharmD,
Sheilyn H Huang,
Shelagh D Schopen,
Shelley H Beausoleil,
Sherri J Jackson,
Sidney B Priesmeyer,
Simone E Pitts,
Sixto M Mercado Rios,
Sonya L Karsik, RAC,
Stacie A Woods,
Stacy A Schneider,
Stephen D Brown,
Stephen T Hansen,
Steven D Kehoe,
Stuart Kim,
Stuart W Russell,
Sudipan Karmakar,
Sue Lee Chan,
Sundy V Sedwick,
Sunita Masson,
Sunita Vij,
Sunitha K Rajaram, PhD,
Susan M Jackson,
Susan T Hadman,
Taichun Qin, PhD,
Tamala P Bogan,
Tamala P Magee,
Tara L Greene,
Tara M Pianko,
Terrence Gee,
Terri L Dodds,
Terry A Hook, DPh,
Terry L Boles,
Thao T Kwan,
Thea C Grome,
Thomas C Mclean, CFS,
Thomas J Arista,
Thuy T Nguyen, LCDR,
Tiara Nbrown Crosen,
Timothy C Grome,
Timothy T Kapsala,
Torrance J Slayton,
Tracey T Duong,
Tracy K Li,
Truong Xuan Nguyen (Andy),
Uduak M Inokon,
Uttaniti Limchumroon (Tom),
Valerie L Whipp,
Vickie L Anderson,
Vijaya L Simhadri,
Vilmary Negron Rodriguez,
Virgilio F Pacio, CSO,
Walden H Lee,
Wayne E Seifert,
Wilfred J Omoloh,
Willemsj,
William J Leonard,
William V Millar,
Willis L Hicks,
Xiaokuang Lai, PhD,
Xiaoping Guan,
Yi Wang, PhD,
Youkeun Kim,
Yumi J Hiramine,
Yvette Mlacour Davis,
Zachary A Bogorad,
Zonglin Hu,
Zuben E Sauna, PhD
Stephanie A Slater, MS's Documents
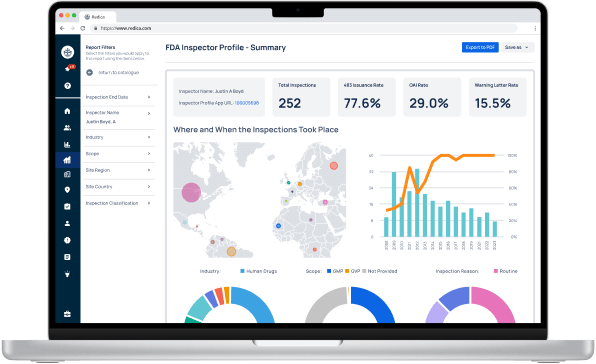
Experience Redica Systems’ NEW investigator profiles and dashboards
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more