FDA Investigator Sonya M Edmonds
Sonya M Edmonds has conducted inspections on 61 sites in 6 countries as of 10 Apr 2024. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
61
Last Inspection Date:
10 Apr 2024
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
India,
Spain,
Japan,
Korea (Republic of),
Hungary
FDA Investigators that have inspected at least one site in common with Sonya M Edmonds:
Abdollah Koolivand,
Adam R Cooke,
Alicia M Mozzachio,
Alifiya H Ghadiali,
Amy Devlin, PhD,
Amy H Ruble,
Anastasia M Shields,
Ann B Borromeo,
Arsen Karapetyan,
Ashutosh Rao, PhD,
Babatunde D Babalola,
Barbara M Frazier,
Bernadette T Tallman,
Betsy C Galliher,
Bo Chi, PhD,
Bonita S Chester,
Brandon C Heitmeier,
Brandy D Brown,
Brentley S Collins,
Brett D Weed,
Brett Weed,
Brittny C Cargo,
Burnell M Henry,
Carl A Huffman, III,
Cassandra L Abellard,
CDR Ileana Barreto Pettit,
CDR Thomas R Berry, PPh,
Charanjeet Jassal,
Charles Yuanchia Kuo, PhD,
Chris Alexander,
Christian D Lynch (CDL),
Christopher Downey, PhD,
Christopher J Adams,
Christopher S Keating,
Claudette D Brooks,
Clifton L Randell,
Constance Y Fears,
Cynthia Jim, CSO,
Daniel C Kearns,
Daniel Lagasse,
Daphne Santiago, PhD,
Darryl T Newell,
David E Lowe,
David Williams,
Davinna Ligons, PhD,
Deborah A Greco,
Demario L Walls,
Denise M Digiulio,
Desiree D Clark,
Devaughn Edwards,
Deyaa Shaheen,
Diane E Bruce,
Diane P Goyette,
Doan T Nguyen, PharmD,
Dorothy P Kramer,
Dr. Jason R Caballero,
Dr. Sriram Subramaniam, PhD,
Dr. Zhihao Qiu (Peter), PhD,
Edward Deberry,
Edward Lukeman,
Eileen J Bannerman,
Ennan Guan,
Ephrem Hunde, PhD,
Eric S Weilage,
Esther C Broner, PhD,
Fabian Nchaparro Rodriguez,
Frank Wackes,
Frederick F Razzaghi,
Gabriel R Mclemore,
Gam S Zamil,
Gamal A Norton,
Gary E Coleman, Jr, REHS,
Gene D Arcy,
George J Flynn,
Gideon N Esuzor,
Gregory Price,
Hai Lient Phung,
Hala L Selby,
Ibad U Khan,
Inga M Warr,
Jacek Cieslak, PhD,
Jacqueline Mdiaz Albertini,
James A Crim,
James P Lewis, Jr,
Jamie L Port,
Jared P Stevens,
Jason F Chancey,
Jason K Morgan,
Jawaid Hamid,
Jee Chung, PhD,
Jeff M Uriarte,
Jennifer Lalama,
Jessica L Pressley,
Joanne E King,
Joanne T Gabay,
John A Gonzalez,
John E Aghadia,
Johnson,
Jose M Cayuela,
Joseph F Owens,
Julie D Bringger,
June P Page,
Karen Takahashi,
Kassa Ayalew, MD,
Kathleen D Culver,
Katrina B Mosley Sloan,
Kemejumaka N Opara,
Khalid M Khan,
Kshitij A Patkar,
Lareese K Thomas,
Larry K Hampton,
Latorie S Jones,
Laurie B Frazier,
Laurie P Norwood,
Lauryl A Smith,
Leo J Lagrotte,
Leopold Kong, PhD,
Lesley K Satterwhite,
Leslie Arivera Rosado, PhD,
Libia M Lugo,
Lillie D Witcher,
LT Daveta L Bailey,
LT Tamara J Henderson,
Luis A Carrion,
Luis Mburgos Medero,
Marcia A Worley,
Margaret M Annes,
Marie F Morin,
Marisa E Stock,
Marla A Cassidy,
Marvin D Jones,
Mary Jeanet Mcgarry,
Mary Kaulee,
Melanie M Walker,
Metitia G Sanders,
Metitia M Gramby,
Michael F Skelly, PhD,
Michelle D Haamid,
Narong Chamkasem, PhD,
Nicole A Lloyd,
Nicole E Knowlton,
Nicole M Bell,
Noreen Muñiz,
Nydia E Colon,
Patricia F Hughes, PhD,
Paul A Bonneau,
Paul L Bellamy,
Paul L Figarole, Jr,
Paula A Trost,
Penny H Mccarver,
Perry H Gambrell,
Philip F Istafanos, DMV, MS,
Postelle Birch Smith,
Prajakta A Varadkar,
Pratik S Upadhyay, DDC,
R Edwadebey Deberry,
Rachael A Moliver,
Rachael L Cook,
Randy L Clarida,
Rashonda N Rucker,
Reba A Gates,
Rebecca Rodriguez,
Regan T Harp,
Richard L Bartlett,
Robert C Coleman,
Robert D Tollefsen,
Robin N Goins,
Rose Xu,
Samantha J Bradley,
Samuel L Murray,
Sarah L Holliday,
Saundrea A Munroe,
Sayeeda Hdabe,
Scott T Ballard,
Seneca D Toms,
Sharna D Pratt,
Simon Williams, PhD,
Song Y Lavalais,
Srinivas R Chennamaneni, PhD,
Steven D Dittert,
Steven Fong, MS, PhD,
Steven M Weinman,
Susan T Hadman,
Susan W Ting,
Suzanne N Vallez,
Tajah L Blackburn,
Tamara M Casselman,
Tamara M Felton,
Tammara A Stephens,
Tammy T Brown,
Teresa C Thompson,
Thomas J Arista,
Thuy T Nguyen, LCDR,
Tim Lee,
Timothy T Kapsala,
Tracy R Ball,
Trang N Cox,
Travis S Bradley,
Tsedenia Woldehanna,
Veronica Fuentes, MS,
Vilmary Negron Rodriguez,
Vincent Thomas,
Viviana Matta,
Wayne S Fortenberry,
Wendy G Tan, PhD,
Xiaoxiao Pan, PhD,
Xiomara Copeland,
Xuhong Li,
Yasamin Ameri,
Young Kim,
Yuan Chia Kuo,
Yun Wu, PhD,
Zada L Giles,
Zhenzhen Liu, PhD
Sonya M Edmonds's Documents
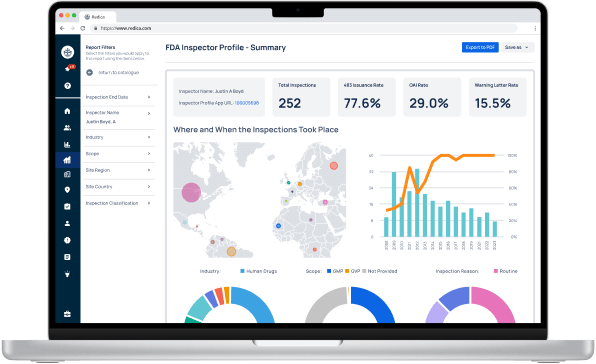
Experience Redica Systems’ NEW investigator profiles and dashboards
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more