FDA Investigator Janete F Guardia
Janete F Guardia has conducted inspections on 227 sites in 19 countries as of 24 Jun 2024. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
227
Last Inspection Date:
24 Jun 2024
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
India,
Germany,
United States of America,
Japan,
China,
France,
Bulgaria,
Malaysia,
Turkey,
Switzerland,
Costa Rica,
Sweden,
Italy,
United Kingdom of Great Britain and Northern Ireland,
Thailand,
Belgium,
Korea (Republic of),
Colombia,
Ireland
FDA Investigators that have inspected at least one site in common with Janete F Guardia:
Aditi S Thakur,
Akbar J Zaidi,
Alanna L Mussawwir Bias,
Alia Legaux,
Alice C Silva,
Allen F Hall,
Althea A Williams,
Alysia M Salonia,
Amanda B Athey,
Amanda E Lewin, PhD,
Amanda Jones,
Andrace L Deyampert,
Andrea A Branche,
Andria L Kuhlman,
Anita Narula, PhD,
Anita R Michael,
Anthony C Warchut,
Anthony E Keller, RPh,
Anthony N Onianwa,
Arindam Dasgupta, PhD,
Ashley M Whitehurst,
Atricia L Irons,
Atul J Agrawal,
Azza Talaat,
Barbara A Rusin,
Barbara D Wright,
Barbara J Holladay,
Barbara J Rincon,
Barbara Janine Breithaupt,
Barbara M Frazier,
Bonita S Chester,
Brenna L Macdowell,
Camille D Brown,
Carl E Lovrich,
Catherine J Laufmann,
CDR Sean T Creighton,
Charles D Brown,
Charles L Larson,
Charles M Kerns, BLT DO,
Cheryl D Mccall,
Christina D Mello,
Christopher D Rush,
Christopher Janik,
Christopher M Elliott,
Christopher R Richardson,
Clotia Cabbey Mensah,
Connie R Kiessling,
Courtney N Long,
Craig A Garmendia,
Creighton T Tuzon,
Cynthia A Harris, MD, RN,
Cynthia F Kleppinger, MD,
Cynthia T Cain,
Daniel J Lahar,
Daniel L Aisen,
Danny D Horner,
Darin S Wiegers,
David J Gallant,
David L Chon,
David P Rice, Jr,
David P Vanhouten,
David R Heiar,
Dawn C Olenjack,
Dawn M Braswell,
Debara R Reese,
Deborah A Greco,
Denise L Burosh,
Dennis Cantellops Paite,
Diane C Thibodeau,
Dien N Nguyen,
Dolores Harper,
Dominic R Frasca,
Donna Ltartaglino Besone,
Dorothy W Lee,
Douglas S Joslin,
Dr. Gopa Biswas, PhD,
Dr. Mark J Seaton, PhD,
Dr. Sriram Subramaniam, PhD,
Dr. Zhou Chen (nmi), MD PhD,
Eduardo L Rodriguez,
Edward D Mcdonald,
Edward J Janik,
Edward P Potter,
Edwin Melendez,
Eileen J Bannerman,
Elizabeth B Griffin,
Ellen J Tave,
Elvis D Morton,
Emest F Bizjak,
Eric C Schmitt,
Eric M Mueller, PharmD,
Erich P Garger,
Erin L Mcfiren,
Ernest Serna, III,
Esaw,
Esteban Beltran,
Evelyn Taha,
Farhana Khan,
Felix J Marrero,
Francis J Eng,
Frank J Marciniak,
Gajendiran Mahadevan, PhD,
Gale E Ryan,
Gale L Glinecki,
Gamal A Norton,
Gene D Arcy,
Geoffrey K Kilili,
Gilbert Valdez, RS,
Gisselle I Sensebe,
Gloria J Baca, MS,
Gregson A Joseph,
Habacuc V Barrera,
Hasan A Irier, PhD,
Hector Cerros,
Hector Jcolon Torres,
Helen B Ricalde,
Himanshu Gupta, PhD,
Holly S Simms,
Iram R Hassan, PhD,
Iris C Macinnes,
J Elkins,
Jacqueline S Warner,
James M Simpson,
James R Fleckenstein,
James R Montero,
James W Leonette,
James W Plucinski,
James W Whitney,
Jamie D Swann,
Jamie M Bumpas,
Jane M Kreis,
Janet B Abt,
Jay M Patria,
Jeffery A Hangartner,
Jeffrey A Reed,
Jeffrey J Thibodeau,
Jeffrey P Raimondi,
Jeffrey R Wooley,
Jennifer C Adams,
Jennifer Cunningham,
Jeremy L Stukes,
Jerry Lott,
Jesse P Hardin,
Jessica D Nanini,
Jessica Fierros, RS,
Jill J Tillman,
Joan T Briones,
Joanne E King,
Joanne M Schlossin,
Jocelyn C Turner,
Jocelyn E Massey,
Joel D Hustedt,
Joel D Martinez,
Joey C West,
Johann M Fitch,
John A Hollings,
John A Iwen,
John A Sciacchitano,
John Dan,
John J Thibodeau,
John P Shelton,
Johnson,
Jonathan B Lewis,
Jonee J Mearns,
Joppie,
Jose A Lopez,
Jose Acruz Gonzalez,
Joseph A Morkunas,
Joseph L Despins, PhD,
Joseph W Matthews,
Joshua D Levin,
Joy P Matthias,
Joyce A Kellett,
Juanita Banuelos,
Jude C Dikes,
Justin A Hefferman,
Jyoti B Patel, PhD,
K Gonzlez,
Kara A Scheibner, PhD,
Karen A Spencer,
Karen L Kosar,
Katarzyna Plona,
Katlin N Stubbs,
Kellia N Hicks,
Kellie L Thommes, RN,
Kelvin Cheung,
Kent A Conforti,
Kevin A Gonzalez,
Kimberly M White,
Kristin N Longfellow,
Kwong P Lee,
Lakecha N Lewis,
Lance Mde Souza, MBA,
Latorie S Jones,
Lauren M Lawrance,
Laurie B Frazier,
LCDR Angelica M Chica,
Leighton Kn Gai,
Leonard H Lavi,
Li Hongpaul Yeh, PhD,
Libia M Lugo,
Linda A Gregory Duty,
Linda R Kuchenthal,
Lirissia Mccoy,
Lisa Hayka,
Lisa M Lopez,
Lisa P Oakes,
Loretta Nemchik,
Lori A Gioia,
Lori S Lawless,
Louis Christy,
Luis A Salinas,
Luis Mburgos Medero,
Lyndsay M Perryman,
Makini Cobourne Duval, PhD,
Marc R Dickens,
Marc S Neubauer,
Marcia G Evans,
Margaret M Annes,
Margarita Santiago,
Marijo B Kambere, PhD,
Marilyn M White,
Mark R Mcclain,
Maryam Tabatabaie,
Matthew B Casale,
Matthew C Watson,
Matthew M Henciak,
Maura Rooney,
Maya M Davis,
Megan A Haggerty,
Meisha A Mandal,
Melanie W Pishnery,
Meredith L Sheridan,
Merideth K Rose,
Miah Jung,
Miaja Umaedi,
Michael F Skelly, PhD,
Michael G Mayfield,
Michael J Nerz,
Michael J Pasternak,
Michael P Anthony,
Michael R Goga,
Michelle J Hines,
Michelle M Noe Varga,
Miguel A Martinez Perez,
Mikayla K Deardorff,
Mike M Rashti,
Milan S Mcgorty,
Mildred L Mccray,
Minh D Phan,
Mohamad A Chahine,
Mohsen Rajabi Abhari, FDA,
Monique Rac Lester,
Msdap Gonzlezk,
Muralidhara B Gavini, PhD,
Myra L Gemmill,
Nadeem I Chaudhry,
Nasser Yazdani, DVM, MPH,
Natasha A Dezinna,
Nathan R Moon,
Nicholas C Mendiola,
Nicholas P Schaub,
Nicholas Z Lu,
Nicola M Fenty Stewart,
Norman Wong,
Osama A Khatib,
Osama M Hamoud,
P John Kamthong,
Pamela N Agaba,
Parul M Patel,
Patricia A Homola,
Patricia A Mcilroy,
Paul L Figarole, Jr,
Paula J Bretz,
Pauline N Logan,
Perry H Gambrell,
Peter E Baker,
Peter R Lenahan,
Philip W Beck,
Quynh H Strandberg,
Rafael E Arroyo,
Rebecca A Coleman,
Rebecca B Welch,
Rebecca E Dombrowski,
Rebecca T Davis,
Regina T Brown,
Rene R Ramirez,
Richard L Rutherford,
Richard Ledwidge (nmi), PhD,
Richard W Berning,
Richmond K Yip,
Robert T Lorenz,
Ronda Rloyd Jones,
Rosa M Santiago,
Rosanna M Goodrich,
Rose Ashley,
Rose Ljean Mary,
Roy Baby,
Ruben C Ayala, PharmD,
Rupa Pradhan,
S Lori Brown, PhD MPH,
Saijal P Naik,
Sam H Haidar, PhD,
Samir C Gala,
Sandra A Hughes,
Sandra S Saniga,
Scott B Laufenberg,
Scott R Nichols, PhD,
Shafiq S Ahadi,
Sharon K Gershon,
Sherri J Jackson,
Sherrie L Krolczyk,
Slater K Bartlett,
Solomon Yimam (SY),
Sony Mathews,
Sripal R Mada, PhD,
Staci G Mcallister,
Stanley B Eugene, BS, BME,
Stephanie Mangigian, MS/OSH, RN,
Stephen C Smith,
Stephen D Eich,
Stephen L Beekman,
Stephen R Souza,
Steven A Gonzales,
Steven R Ziser,
Susanne M Richardson, MS RAC,
Tamara N Champion,
Tanya R Syffrard,
Tawny L Colling,
Teddy Tom,
Terri E Gibson,
Thai T Duong,
Thea C Grome,
Theressa B Smith,
Thomas J Arista,
Thomas W Gordon,
Timothy B Webb,
Timothy C Grome,
Timothy M Albright,
Todd D Alspach,
Todd J Maushart,
Tomika L Crafter,
Tonia F Bernard,
Tony T Yang,
Tracy R Ball,
Tricia S Martinez,
Troy R Petrillo,
Valeria A Moore,
Valerie G Vallely,
Ve Garcia,
Victoria M Daddeo,
Vivian Garcia,
Wendy A Johnecheck,
William H Linkroum,
Xiaohan Cai, PhD,
Xiaojun Yan,
Xiaokuang Lai, PhD,
Xikui Chen (nmi), PhD,
Yaw O Osei,
Yiyue Zhang (nmi), PhD,
Young M Choi, PhD,
Zachary A Bogorad,
Zachary L Miller,
Zerita White,
Zhongren Wu
Janete F Guardia's Documents
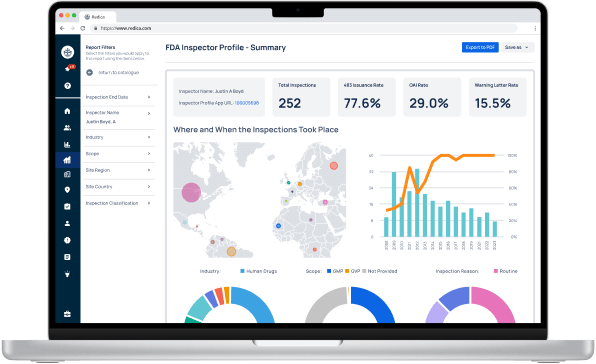
Experience Redica Systems’ NEW investigator profiles and dashboards
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more