FDA Investigator Susan F Laska, MS
Susan F Laska, MS has conducted inspections on 154 sites in 16 countries as of 22 Sep 2014. See below for a list of the FDA enforcement documents resulting from those inspections.
Investigator Details
Number of Inspected Sites:
154
Last Inspection Date:
22 Sep 2014
Investigator Role:
FDA Investigator
Redica ID:
Countries of Inspections:
United States of America,
Austria,
Ireland,
France,
China,
Poland,
Canada,
Sweden,
Italy,
Spain,
Norway,
Germany,
United Kingdom of Great Britain and Northern Ireland,
Switzerland,
Belgium,
Denmark
FDA Investigators that have inspected at least one site in common with Susan F Laska, MS:
Aaron L Dunbar,
Alan D Centi,
Alan L Truong,
Alan P Kurtzberg,
Albert A Salvador,
Alexander M Kay,
Alice S Tsao,
Alicia M Mozzachio,
Alison N Correll,
Alla Kachko, PhD,
Allen F Hall,
Althea A Williams,
Amber D Brodas,
Amy N Chen,
Anastasia I Offordile,
Anastasia I Onuorah,
Anastasia M Shields,
Andrace L Deyampert,
Andrea D Swingle,
Angela K Shen,
Anissa M Cheung,
Anita Narula, PhD,
Anita R Michael,
Ann L Demarco,
Ann Marie Karnick,
Ann Marie Montemurro,
Ann Marie Schofield,
Anna Lazar,
Anthony A Charity,
Antonina Aydanian,
Ariel Cruz Figueroa,
Arthur S Williams, Jr,
Ashar P Parikh,
Ashley L Reiber,
Astrida B Mattson,
Audrey Thereset Uy,
Azza Talaat,
Barbara J Rincon,
Barbara Janine Breithaupt,
Barbara M Frazier,
Bei Y He,
Benjamin J Smith,
Bijoy Panicker,
Bo Chi, PhD,
Bonita S Chester,
Bradley Dworak, PhD,
Brandy N Lepage,
Brentley S Collins,
Brian D Nicholson,
Brian D Young,
Brian S Keefer,
Brittany D Terhar,
Burnell M Henry,
Byungja E Marciante,
Calvin B Koerner,
Camerson E Moore,
Cara M Minelli,
Carl Lee,
Carla A Norris,
Carla J Lundi,
Carla Lankford, MD, PhD,
Carol S Sanchez,
Caryn M Mcnab,
Cassandra Overking,
CDR Donald Ertel,
CDR Jeremy L Wally, PhD,
Chao Ming Tsai,
Chaoying C Ma,
Charisse K Green,
Charles I Ann,
Charles Jewell,
Charles L Zhou,
Charles M Edwards,
Charles R Bonapace, PhD,
Charles R Cote, RIC,
Charlotte E Wilkins,
Chris A Sack,
Christian D Lynch (CDL),
Christina J Sauder, PhD,
Christina K Theodorou,
Christine A Harman, PhD,
Christopher D Leach,
Christopher J Adams,
Chunsheng Cai, PhD,
Colleen M Damon,
Concepcion Cruz, Jr,
Connie P Rezendes,
Constance Lewin, MD,
Constantin Y Philopoulos,
Corrine M Carter,
Craig D Zagata,
Crystal A Harlan,
Cynthia A Palmer,
Cynthia J Lee, MS,
Cynthia Jim, CSO,
Dana M Klimavicz,
Dandan Wang, PhD,
Daniel R Tammariello,
Daniel T Lee,
Daniel W Cline,
Daniel W Johnson,
Dave Hafner,
David A Oluwo,
David A Paterson,
David Frucht, MD,
David J Gomes,
David J Hafner,
David J Hapner,
David L Pearce,
David S Cho, PhD,
Dawn E Barkans,
Debara R Reese,
Debara Reese,
Deborah J Parris,
Debra J Bennett,
Debra L Pagano,
Demitria J Argiropoulos,
Demitria J Xiradakis,
Denise L Burosh,
Denise M Digiulio,
Dennis Cantellops Paite,
Dennis E Guilfoyle, PhD,
Derek S Dealy,
Deyaa Shaheen,
Diane L Raccasi,
Dina A Tallman,
Djamila Harouaka,
Dolores E Price,
Don H Bark, PhD,
Donald C Obenhuber, PhD,
Donald L Lech,
Dr. Chunchang Fang,
Dr. Gerald E Poly,
Dr. Jason R Caballero,
Dr. Mark J Seaton, PhD,
Dr. Sriram Subramaniam, PhD,
Dr. Zhihao Qiu (Peter), PhD,
Edmund F Mrak, Jr,
Edward D Mcdonald,
Edwin Martinez,
Edwin Melendez,
Eileen A Liu,
Elaine E Gilfillan,
Ellen Huang,
Emest F Bizjak,
Emily J Orban,
Eric S Myskowski,
Eric S Weilage,
Erika V Butler,
Esther C Broner, PhD,
Farhana Khan,
Felix Maldonado,
Francis A Guidry,
Frank W Perrella,
Freyja V Lynn,
Gary L Pierce,
Gayle S Lawson,
Gene D Arcy,
George Pyramides,
Gerard Pde Leon,
Ginger M Sykes,
Gloria J Baca, MS,
Gwyn G Dickinson,
H Ljamilla Selby,
Hala L Selby,
Hala Lj Whetstone,
Haruhiko Murata,
Heika R Bounds,
Heika R Tait,
Helen B Ricalde,
Heriberto Negron Rivera,
Hung H Do, MS,
Iris C Macinnes,
Ivis L Negron,
J David Doleski,
Jacqueline A O'shaughnessy, PhD,
Jacqueline Mdiaz Albertini,
Jacqueline S Warner,
Jacquelyn Gess,
Jaison J Eapen,
James C Illuminati,
James C Maclaughlin,
James I Giefer,
James L Dunnie, Jr,
James M Mason,
James P Mcevoy,
James R Evans,
James R Fleckenstein,
Jason F Chancey,
Javier E Santos,
Jean Blackston Hill,
Jeen S Min,
Jeffrey A Sommers,
Jeffrey D Meng,
Jeffrey P Raimondi,
Jennifer A Kemp,
Jennifer D Hollstrom,
Jennifer L Bridgewater,
Jennifer M Gogley,
Jessica L Pressley,
Jessica S Estriplet,
Joan A Loreng,
Joan Adamo,
Joanne E King,
Joanne Heim,
Joanne M Hajoway,
Joel D Hustedt,
Joey V Quitania,
John A Gonzalez,
John D Finkbohner, PhD,
John D White,
John M Mcinnis,
John M Mclnnis,
Jonathan G Matrisciano,
Jose A Lopez,
Jose Acruz Gonzalez,
José E Meléndez,
Jose R Hernandez,
Joseph A Piechocki,
Joseph George,
Joseph L Despins, PhD,
Joseph R Lambert,
Joseph R Strelnik,
Joseph S Fanelli,
Joseph T Dougherty,
Josh Renzo N Ramilo,
Joshua C Schafer,
Julianne C Mccullough,
Julie D Bringger,
Junho Pak,
Justine Tomasso,
Karen A Briggs,
Karen E D'orazio,
Karen L Kosar,
Karen M Montgomery (KMM),
Karen Takahashi,
Kari M Johansen,
Karthik B Iyer,
Karyn M Campbell,
Katherine E Jacobitz,
Kathleen B Mihalik,
Kathryn A Krentz,
Kathryn Carbone, MD,
Kathryn E Ogger,
Kelly N Kerr,
Kelvin Cheung,
Kendra L Brooks,
Kenneth H Williams,
Kenneth M Gordon,
Kenneth O Gee, PhD,
Kent C Faul,
Kevin A Gonzalez,
Kevin D Kallander,
Kevin M Maguire,
Kham Phommachanh,
Khin Mang U, MD,
Kim Lthomas Cruse,
Kimberley A Hoefen,
Kimberly Lewandowski Walker,
Ko U Min,
Kristen D Evans,
Kristin M Abaonza,
Kristina J Donohue,
Kristina L Conroy,
Kristina Mjoyce Pittman,
Kristy A Zielny,
L'oreal F Walker,
Larry K Austin,
Lata C Mathew, PhD,
Laura Fontan, MS,
Laurel A Beer,
Lauren Iacono Connors, PhD,
Laurie P Norwood,
LCDR Debra Emerson,
LCDR Margaret Edi Gennaro,
LCDR Susan E Polifko,
Leigh Anne Myers,
Lewis K Antwi,
Libia M Lugo,
Linda M Hoover,
Linda Thai,
Lisa B Orr,
Lisa K Capron,
Lisa M Bellows,
Lisa M Feola,
Lisa P Oakes,
Lixia Cai,
Lloyd A Kinzel,
Lori Peters,
LT John M Mastalski,
LTJG Bradley E Benasutti,
Lucas B Leake,
Luella J Rossi,
Luis A Dasta,
Luis Mburgos Medero,
Lynda L Perry, PhD,
Maida Henesian (NMI),
Marcellinus D Dordunoo,
Marcus A Ray,
Marcus F Yambot,
Marea K Harmon,
Margaret A Smithers,
Margaret M Annes,
Maria A Reed,
Maria Joselopez Barragan, PhD,
Maria Pkelly Doggett, MBA,
Marian E Major, PhD,
Marie F Morin,
Marijo B Kambere, PhD,
Marion Michaelis,
Mariza M Jafary,
Marjorie A Shapiro, PhD,
Mark A Elengold,
Mark R Mcclain,
Mark W Babbitt,
Marlene G Swider,
Marsha W Major,
Martha T Olone,
Martin K Yau, PhD,
Mary Davis Lopez,
Marybet Lopez Negron,
Massoud Motamed,
Mate Tolnay,
Matthew A Spataro,
Matthew B Casale,
Matthew D Schnittker,
Matthew R Noonan,
Maureen A Wentzel,
Maxine H Wong,
Meena Bansal Gupta, PhD,
Meisha R Sampson,
Meisha Waters,
Melina Lrodriguez Upton,
Melissa D Ray,
Melissa J Garcia,
Melissa M Dauksis,
Mercy Oyugi,
Michael A Charles,
Michael A Taylor,
Michael Curbarg,
Michael D Omeara,
Michael E Maselli,
Michael F Skelly, PhD,
Michael Gurbarg,
Michael J Kuchta,
Michael J Nerz,
Michael L Casner,
Michael R Goga,
Michael Shanks, MS,
Michele Gottshall,
Michele L Forster, PhD,
Michele L Obert,
Michele M Falchek,
Michele Perry Williams,
Mihaly S Ligmond,
Mike M Rashti,
Mikel T Wright,
Milan S Blake,
Mindy M Chou,
Minh D Phan,
Monica M Mcclure,
Montgomery Montgomery, Karen M,
Mra B Kamberem,
Mra Davism,
Mra M Barbosar,
Mra Mcculloughj,
Mra Munizn,
Muna Algharibeh,
Muralidhara B Gavini, PhD,
Myra K Casey,
Nadeem I Chaudhry,
Nailing Zhang,
Nancy G Schmidt,
Nancy T Waites,
Nasir Ali, PhD,
Neal L Adams,
Neali H Lucas,
Nibin Varghese, MS,
Nicholas L Paulin,
Nicholas Obiri, PhD,
Niketa Patel,
Nikhil Thakur,
Nilufer M Tampal, PhD,
Noreen Muñiz,
Norman K Starks,
Omotunde O Osunsanmi,
Parul M Patel,
Patricia A Cochran,
Patricia F Hughes, PhD,
Patricia H Dlugosz,
Patrick B Cummings,
Patrick C Klotzbuecher,
Patsy J Domingo,
Paul E Stein,
Paul J Teitell,
Paul L Bellamy,
Paul Lihong Yeh, PhD,
Paul Mouris,
Paul Z Balcer,
Paula A Trost,
Perry T Nichols,
Philip F Istafanos, DMV, MS,
Prabhu P Raju,
Qiao Y Bobo,
Rabin N Ghoshal,
Rafael Nevarez Nieves,
Rafeeq A Habeeb,
Raffi B Papazian,
Rahi D Patel,
Ralph Jerndal,
Ramon E Martinez,
Randy L Self,
Raymond T Oji,
Rebecca E Dombrowski,
Rebecca K Olin,
Rebecca Rodriguez,
Regina T Brown,
Reginald D Neal,
Reyes Candau Chacon, PhD,
Richard E Needham,
Richard H Penta,
Richard K Vogel,
Richard L Friedman,
Richard Ledwidge (nmi), PhD,
Richard W Thornton,
Ridgely C Bennett, MD,
Rita F Larocca Mahoney,
Robert B Shibuya, MD,
Robert C Coleman,
Robert C Steyert,
Robert D Tollefsen,
Robert J Ham,
Robert J Maffei,
Robert Jennings,
Robert L Stevenson,
Robert M Barbosa,
Robert Maffei,
Robin Levis, PhD,
Robin P Mathew,
Rochelle K Kimmel,
Rodney D Combs,
Ronald A Stokes,
Rosanna M Goodrich,
Rose Ashley,
Rose Ljean Mary,
Rowena S Nguyen,
Ruben C Quintana,
Rumany C Penn, PharmD,
Russell J Glapion,
Ruth Moore, PhD,
S Lori Brown, PhD MPH,
Saied A Asbagh,
Sam Pepe,
Samantha J Bradley,
Samuel K Gibbons, Jr,
Samuel T Walker,
Sandra A Boyd,
Sangeeta M Khurana, PhD,
Sangeeta M Rataul,
Sarah B Tanksley,
Sarah Forney,
Scott B Laufenberg,
Scott E Norris,
Scott T Ballard,
Sean R Marcsisin,
Sena G Dissmeyer,
Seneca D Toms,
Sharon K Thoma, PharmD,
Sheila Dreher Lesnick,
Shelby N Turner,
Shelley L Waring,
Shererk,
Shirley H Isbill,
Shirley J Berryman,
Shuang Tang,
Sidney B Priesmeyer,
Simone E Pitts,
Sixto M Mercado Rios,
Sneha S Patel,
Stefanie L Heckman,
Stefanie L Kremer,
Stephanie Mangigian, MS/OSH, RN,
Stephen C Smith,
Stephen D Brown,
Stephen J Koniers,
Steven A Brettler,
Steven A Gonzales,
Steven A Rubin,
Steven C Madzo,
Steven D Kehoe,
Steven P Donald,
Susan A Zullo, PhD,
Susan M Jackson,
Susan P Bruederle,
Susan W Ting,
Suyang Qin,
Syed N Ali,
Syeda N Mahazabin,
Tammy L Chavis,
Tekalign Wondimu,
Temar Q Williams,
Terri L Dodds,
Thomas E Friel,
Thomas J Arista,
Thuy T Nguyen, LCDR,
Timothy T Kapsala,
Tina M Pawlowski,
Tina S Roecklein,
Todd J Maushart,
Tonia L Sawyer,
Torrance J Slayton,
Tracy K Li,
Truong Xuan Nguyen (Andy),
Unnee Ranjan,
Uttaniti Limchumroon (Tom),
Verlinda A Narcisse,
Virgilio F Pacio, CSO,
Viviana Matta,
Vlada Matusovsky,
Walden H Lee,
Warren J Lopicka,
Wayne E Seifert,
Wayne T Smith,
Wei Hua Emily Wu,
Wei Wang, PhD,
William C Brenneman,
William D Tingley,
William J Leonard,
Xiao Wang,
Xiaokuang Lai, PhD,
Xiomara Copeland,
Yangmin Ning,
Yehualashet A Gessesse,
Yumi J Hiramine,
Yvins Dezan,
Yvonne C Mcknight,
Yvonne C Wood,
Zachary A Bogorad,
Zachary L Miller,
Zachary L Stamm,
Zhong Li, PhD
Susan F Laska, MS's Documents
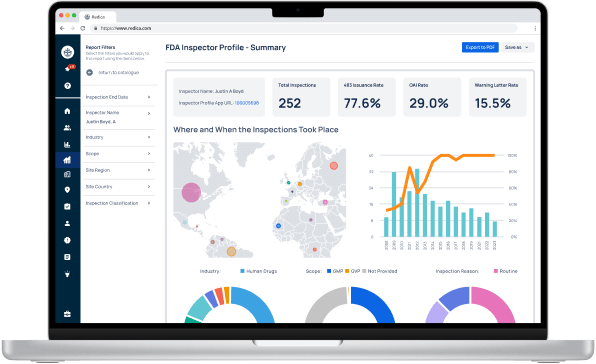
Experience Redica Systems’ NEW investigator profiles and dashboards
Redica Systems customers get access to over 1,400 FDA Investigator profiles, containing powerful analysis like:
- Total inspections conducted
- 483 rate
- OAI rate
- Warning Letter rate
- Inspections by Industry, Scope, and Inspection Reason
- And much more
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Talk to sales to access our investigator profiles
Loading...
Investigator Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to profiles similar to the one above for every current and former FDA Investigator, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more