Amphastar Pharmaceuticals, Inc. [Rancho Cucamonga / United States of America]
Amphastar Pharmaceuticals, Inc. [Rancho Cucamonga / United States of America] located in RANCHO CUCAMONGA CALIFORNIA, United States of America had its last known inspection on 01 Mar 2024. There are 29 known inspections on record.
Site Details
Industries:
Human Drugs,
Medical Devices
Tags:
Human Drugs,
Medical Devices,
Generics,
Clinical Bioequivalence or Bioavailability Study,
Analytical Testing,
FDF Manufacturer,
Manufacturer,
Corporate Headquarters,
Sterile,
Combination Product,
Medical Device Manufacturer,
Medical Devices: Manufacturer
Last Inspection Date:
01 Mar 2024
FEI:
3002936358
Redica ID:
Investigators:
Binh T Nguyen,
James B Arnett,
Srinivas R Chennamaneni, PhD,
Iris C Macinnes,
Lilly O Barton,
Sue Lee Chan,
Kim Lthomas Cruse,
Michael T Cyrus,
Jeffrey P Raimondi,
Jinnie Kokiatkulkij,
Lillian S Wu,
Crystal Monroy,
Marcus F Yambot,
Mark C Saale,
Robert C Coleman,
Ashar P Parikh,
Rachel C Stanton,
Carrie A Hughes,
Elizabeth Ad Girard,
Homero W Aguilar,
David Serrano,
Carla J Lundi,
Ling Yu L Liu,
Philip F Istafanos, DMV, MS,
Yvonne Y Wu,
Tamala P Bogan,
Truong Xuan Nguyen (Andy),
Himanshu Gupta, PhD,
Jennifer M Gogley,
Mark W Babbitt,
Tamala P Magee,
Lakecha N Lewis,
Amir Alavi,
Hossein Khorshidi, PhD,
Sonya L Karsik, RAC,
Amanda L Fyles,
Uttaniti Limchumroon (Tom),
Kham Phommachanh,
James R Fleckenstein,
Bichsa T Tran,
Caryn M Mcnab,
Virgilio F Pacio, CSO,
John A Gonzalez,
Xiaohui Shen,
Walden H Lee
Amphastar Pharmaceuticals, Inc. [Rancho Cucamonga / United States of America]'s Most Recent Documents
| Publish Date | Document Type | Title |
|---|---|---|
| November, 2025 | FDA 483 | Amphastar Pharmaceuticals, Inc. - Form 483, 2025-11-07 |
| March, 2024 | FDA 483 | Amphastar Pharmaceuticals, Inc. - Form 483, 2024-03-01 |
| June, 2021 | FDA 483 | Amphastar Pharmaceuticals, Inc. - Form 483, 2021-06-22 |
| February, 2019 | FDA 483 | Amphastar Pharmaceuticals, Inc. - Form 483, 2019-02-12 |
| March, 2017 | FDA 483 | Amphastar Pharmaceuticals, Inc. - Form 483, 2017-03-31 |
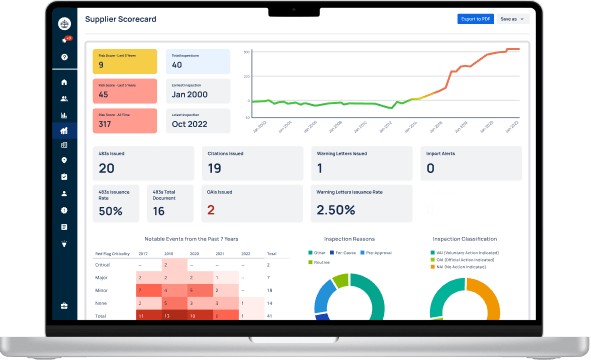
Experience Redica Systems’ NEW site dashboards and profiles
Redica Systems customers can leverage the full Quality and Regulatory Intelligence suite, with powerful analysis covering thousands of sites around the world. You can search by FEI, or more powerfully, by the Redica ID, our universal identifier for global life sciences manufacturing sites, an industry first.
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
Loading...
Site Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to our extensive inventory of site profiles, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more