Intelligent Oversight for Device Quality and Patient Safety
Medical device organizations need visibility across regulatory change, inspections, suppliers, and post-market signals to ensure product safety and maintain global compliance. Redica unifies these intelligence layers into a single, explainable source of truth for actionable insight across the device lifecycle.


















How MedTech Teams Use Redica
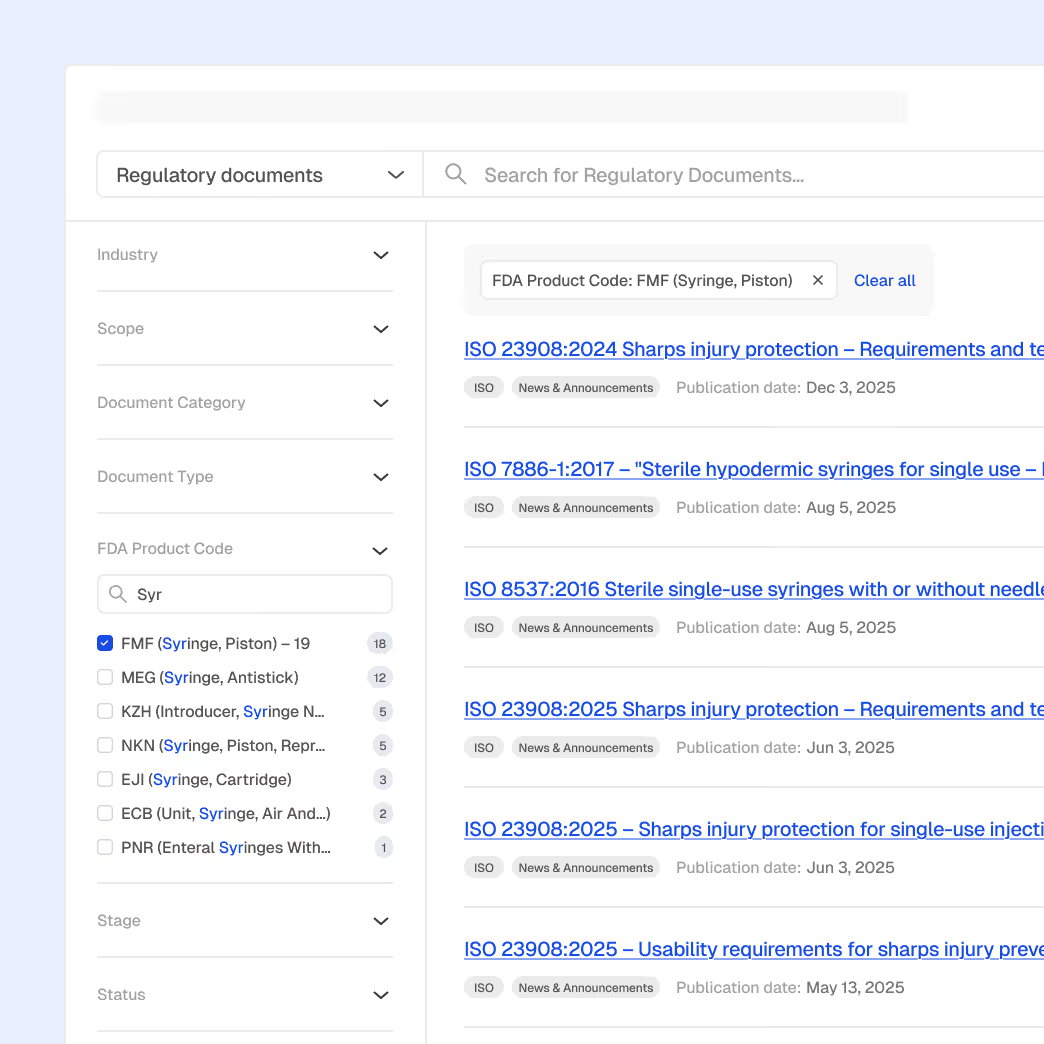
Global Regulatory & Standards Monitoring
Stay aligned with evolving global regulations, standards, and guidance that impact device design, labeling, manufacturing, and post-market obligations.
Monitoring of FDA, EU MDR/IVDR, ISO, and global health authorities
Topic-based filtering for device class, product type, and regulatory area
AI-assisted summaries highlighting relevance and potential impact
Linkage to SOPs and quality processes for change control
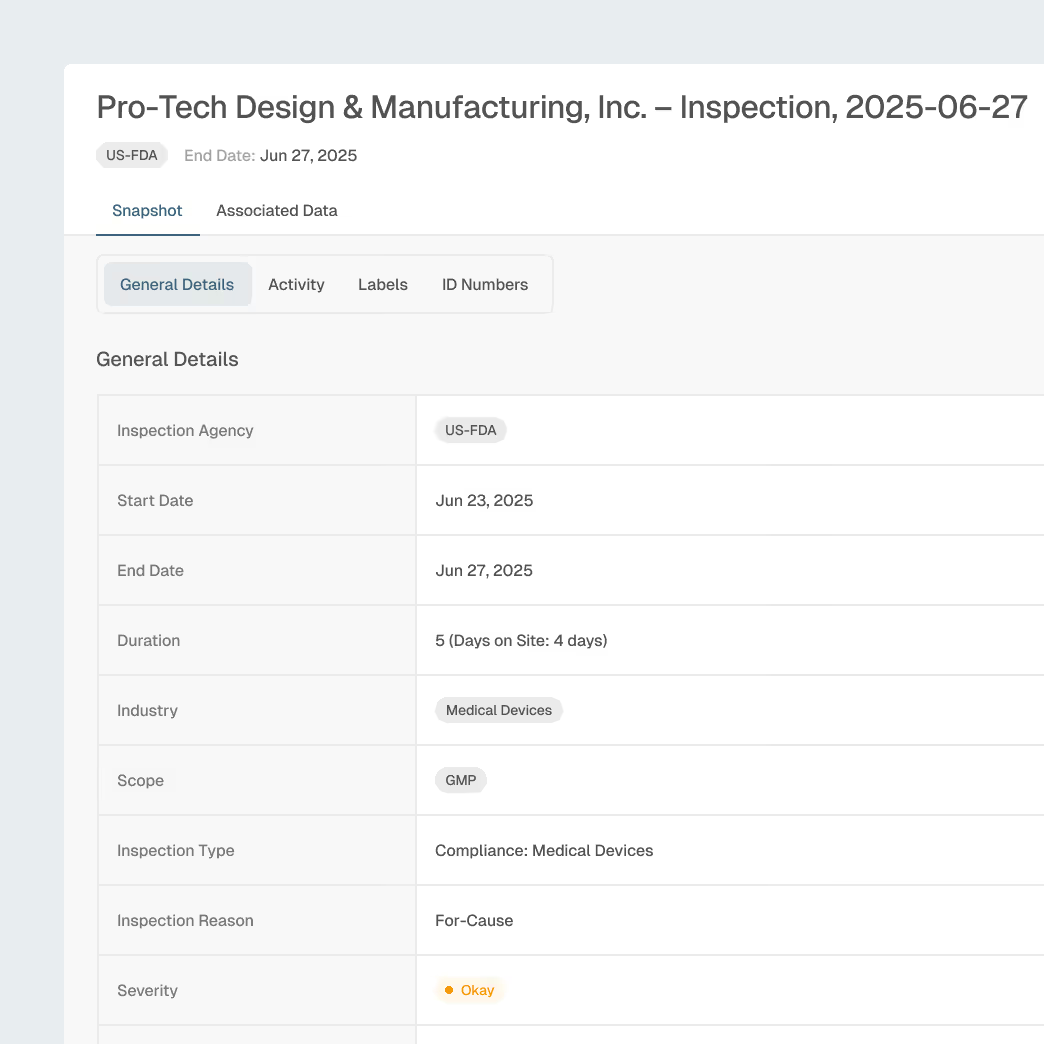
Inspection Readiness & Enforcement Awareness
Prepare manufacturing sites and contract partners with insight into regulator focus areas, inspection trends, and enforcement patterns.
Global inspection and enforcement intelligence
Inspector, firm, and site profiles
Trending observation analysis by device type and region and site profiles
Explainable AI summaries for focused site preparation
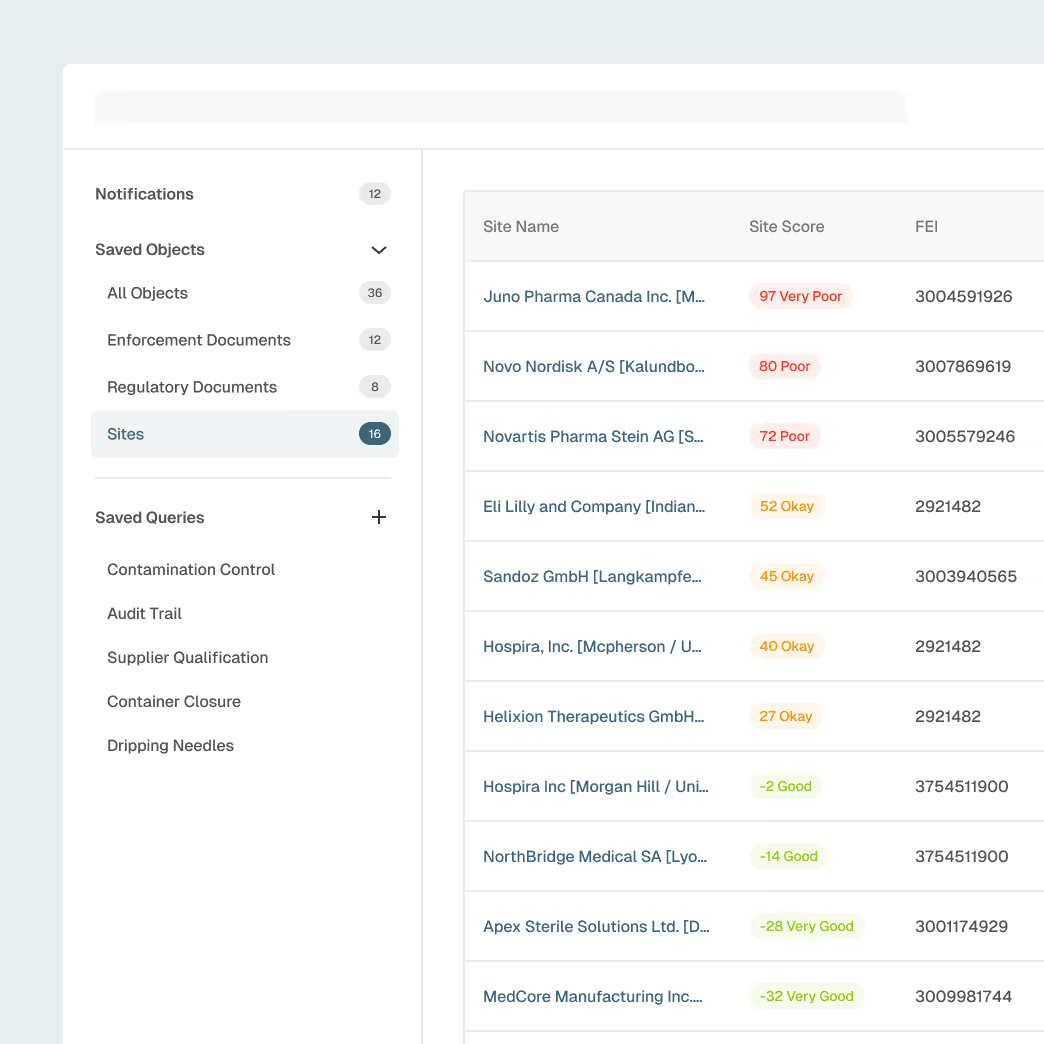
Supplier & Contract Manufacturer Oversight
Strengthen oversight across component suppliers, contract manufacturers, and critical partners with unified visibility into site-level risk.
Site and supplier profiles enriched with inspection history
AI-detected early signals ofquality or compliance risk
Benchmarking to compare supplier performance
Alerts for inspections, warning letters, or enforcement actions
Post-Market Surveillance & Vigilance
Identify emerging safety or quality issues by connecting post-market signals with regulatory and inspection context.
Monitoring of recalls, adverse events, and safety trends
AI-driven clustering of device-related signals
Correlation with inspection and enforcement activity
Unified timelines to support faster triage and escalation
.png)
Why MedTech Companies Choose Redica
Built for the Device Lifecyle
Connect regulatory, inspection, supplier, and post-market signals across design, manufacturing, and commercialization.
Explainable Intelligence for Regulatory Scrutiny
AI-assisted insights include traceable sources and logic to support audits, inspections, and reviews.
Consistency Across Global Operations
Standardized intelligence spans sites, suppliers, regions, and device portfolios.
Embedded in Daily Workflows
Access insights in the Redica App or embed intelligence directly into QMS, RIM, ERP, and supplier systems.
.avif)