Amgen Manufacturing Limited LLC [Ceiba Norte / Puerto Rico]
Amgen Manufacturing Limited LLC [Ceiba Norte / Puerto Rico] located in CEIBA NORTE PUERTO RICO, Puerto Rico had its last known inspection on 05 Jul 2023. There are 33 known inspections on record.
Site Details
Industries:
Medical Devices,
Human Drugs,
Biologics
Tags:
Medical Devices,
Human Drugs,
Analytical Testing,
API Manufacturer,
Packaging,
Label,
Manufacturer,
FDF Manufacturer,
Sterile,
Biologics,
Biologics Manufacturer,
Biologics API Manufacturer
Last Inspection Date:
05 Jul 2023
FEI:
1000110364
Redica ID:
Investigators:
Robert Darius,
Michael Shanks, MS,
Calvin B Koerner,
Rebecca Parrilla,
Patricia F Hughes, PhD,
Milva E Melendez Perales,
Timothy Wadkin, PhD,
Paul Z Balcer,
Jerri Baker,
Jennifer Lalama,
Jose A Lopez,
Omotunde O Osunsanmi,
Michelle Yclark Stuart,
Zhong Li, PhD,
Jose Acruz Gonzalez,
Libia M Lugo,
Nicholas Obiri, PhD,
Maria Gutierrez Lugo, PhD,
Nikolay Spiridonov,
Wendy G Tan, PhD,
Andrea George, PhD,
Arlene M Badillo,
José E Meléndez,
Ivis L Negron,
Riley C Myers, PhD,
Paula A Trost,
Dennis Cantellops Paite,
Zachary L Stamm,
Eliezar Ramos,
Jose Velez,
Rebecca Rodriguez,
Jorge L Lajara,
Myriam M Sosa,
Christopher Downey, PhD,
Michael A Charles,
Nydia E Colon,
Dov H Pluznik, PhD,
Adaliz Santaliz Cruz,
Marian E Ramirez,
Noreen Muñiz,
Alan A Rivera,
Vilmary Negron Rodriguez,
LT Rafael Gonzalez,
Ramon A Hernandez,
Ashley N Queen, PhD,
Steven C Madzo,
Colleen Thomas, PhD,
Rebecca E Dombrowski,
Maxwell Van Tassell, PhD,
Erika V Butler,
Donald C Obenhuber, PhD,
Dr. Zhihao Qiu (Peter), PhD,
Jose R Lopez,
Steven Fong, MS, PhD,
Ramesh Potla, PhD,
Thomas J Arista
Amgen Manufacturing Limited LLC [Ceiba Norte / Puerto Rico]'s Most Recent Documents
| Publish Date | Document Type | Title |
|---|---|---|
| July, 2023 | FDA 483 Response | Amgen Manufacturing Limited LLC - Form 483R, 2023-07-19 |
| July, 2023 | FDA 483 | Amgen Manufacturing Limited LLC - Form 483, 2023-07-05 |
| September, 2021 | FDA 483 | Amgen Manufacturing Limited LLC - Form 483, 2021-09-08 |
| February, 2020 | EIR | Amgen Manufacturing Limited LLC - EIR, 2020-03-17 |
| February, 2020 | FDA 483 Response | Amgen Manufacturing Limited LLC - Form 483R, 2020-03-18 |
Experience Redica Systems’ NEW site dashboards and profiles
Redica Systems customers can leverage the full Quality and Regulatory Intelligence suite, with powerful analysis covering thousands of sites around the world. You can search by FEI, or more powerfully, by the Redica ID, our universal identifier for global life sciences manufacturing sites, an industry first.
Investigator Profiles are not sold as on-demand documents. If you’d like to see one, fill out the form below to connect with our Sales Team and get it in your inbox as little as 24 hours.
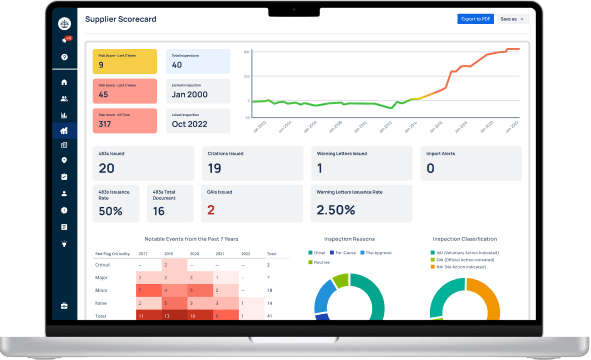
Loading...
Site Profiles Are Just the Tip of the Iceberg
Becoming a Redica Systems customer not only provides you with unlimited access to our extensive inventory of site profiles, but also grants you access to:
Inspection Intelligence
- Pre-Approval Inspection (PAI) trends
- The ability to filter nearly any FDA enforcement action by GxP labels like GMP, GCP, etc.
- Latest 483s: see what the trends are across all FDA Investigators
- CFR Heatmap: Citations aggregated by Subpart by Year
Vendor Intelligence
- Full inspection histories for your vendors down to the specific site level
- All of the functionality mentioned above but isolated to just your vendors
Regulatory Intelligence
- Zero in on the trends for a specific agency/regulator (ex. FDA, MHRA, EMA, Health Canada)
- All the latest "Signals" from agencies around the world, segmented by Country, Type (ex. Guidance, Decree...), Category (ex. Rules/Regulations/Guidance, News, Reports...), Theme (ex. Cell and Gene Therapy, AI...), and more